

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Activation of human ERbeta expressed in human U2-OS cells by luciferase reporter gene assay relative to untreated control | J Med Chem 55: 9607-18 (2012) Article DOI: 10.1021/jm300860j BindingDB Entry DOI: 10.7270/Q2R212H2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
University of Richmond Curated by ChEMBL | Assay Description Activation of human ERbeta assessed as induction of transcriptional activation incubated for 24 hrs by luciferase reporter gene assay | Bioorg Med Chem 27: 2075-2082 (2019) Article DOI: 10.1016/j.bmc.2019.04.003 BindingDB Entry DOI: 10.7270/Q2G73J4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20625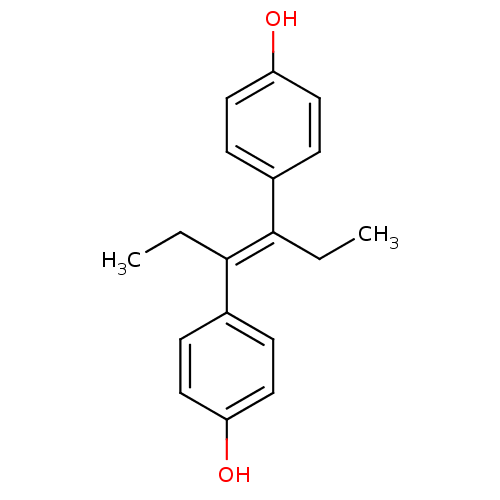 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | 0.0200 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 45: 1399-401 (2002) Article DOI: 10.1021/jm015577l BindingDB Entry DOI: 10.7270/Q27M0665 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128906 BindingDB Entry DOI: 10.7270/Q2S186FF | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at full length ERbeta (unknown origin) after 24 hrs by cell based ERE-driven luciferase reporter gene assay | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at full length human ERbeta receptor assessed as transcriptional activity incubated for 22 to 24 hrs by cell based luciferase report... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115670 BindingDB Entry DOI: 10.7270/Q2ZK5MCF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50561302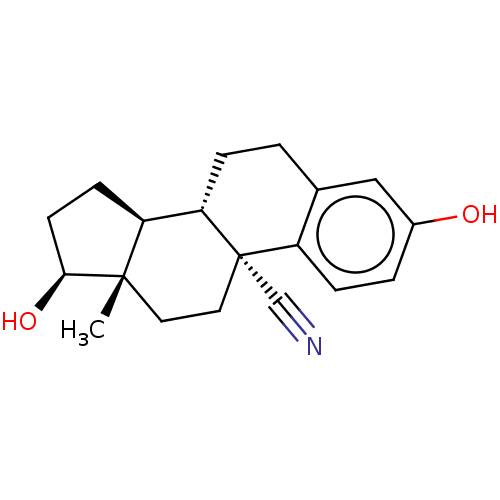 (CHEMBL4782490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at full length human ERbeta receptor assessed as transcriptional activity incubated for 22 to 24 hrs by cell based luciferase report... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115670 BindingDB Entry DOI: 10.7270/Q2ZK5MCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Estrogenic activity at ERbeta (unknown origin) expressed in human MDA-MB-231/beta41 cells after 18 hrs by renilla luciferase reporter gene assay | J Nat Prod 81: 966-975 (2018) Article DOI: 10.1021/acs.jnatprod.7b01070 BindingDB Entry DOI: 10.7270/Q2DV1NKT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
University of Richmond Curated by ChEMBL | Assay Description Activation of human ERbeta assessed as induction of transcriptional activation by luciferase reporter gene assay | Bioorg Med Chem 27: 2075-2082 (2019) Article DOI: 10.1016/j.bmc.2019.04.003 BindingDB Entry DOI: 10.7270/Q2G73J4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Transcriptional activation of ERbeta receptor expressed in CHO-K1 cells after 24 hrs by luciferase reporter gene assay | Eur J Med Chem 54: 188-96 (2012) Article DOI: 10.1016/j.ejmech.2012.04.041 BindingDB Entry DOI: 10.7270/Q2445NJ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor Beta | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558578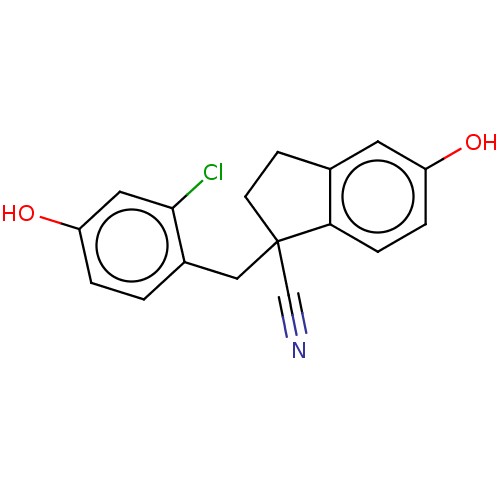 (CHEMBL4753115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | 0.0700 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 45: 1399-401 (2002) Article DOI: 10.1021/jm015577l BindingDB Entry DOI: 10.7270/Q27M0665 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in HEK293 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 5265-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.053 BindingDB Entry DOI: 10.7270/Q2S46TKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Transcriptional activation of estrogen receptor beta in U2OS cell using estrogen-regulated luciferase reporter gene plasmid upon incubation for 20-30... | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558585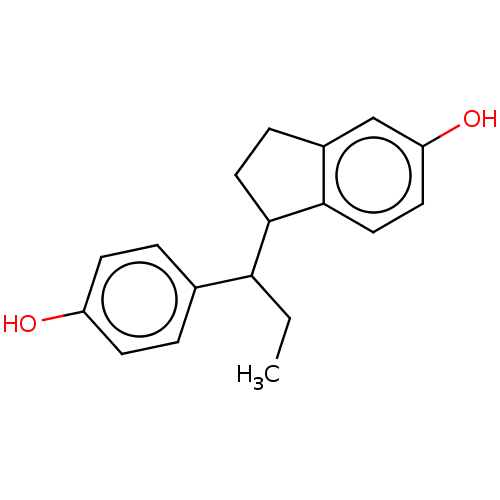 (CHEMBL4740602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Universita di Pisa Curated by ChEMBL | Assay Description Agonist activity at full-length human estrogen receptor beta expressed in human HEC1 cells assessed as transcriptional activation after 24 hrs by luc... | J Med Chem 52: 858-67 (2009) Article DOI: 10.1021/jm801458t BindingDB Entry DOI: 10.7270/Q23R0SRH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106634 ((S)-3-(4-Hydroxy-phenyl)-2-((R)-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor Beta | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558586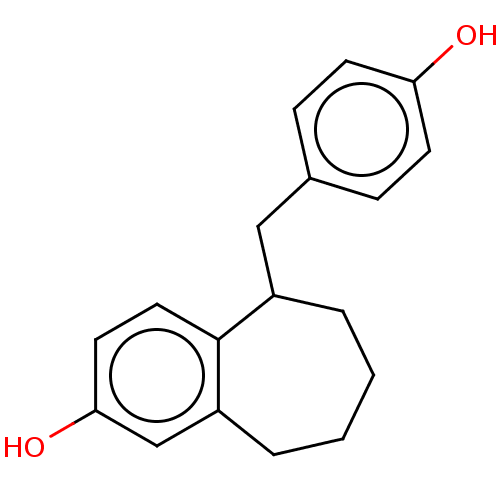 (CHEMBL4795195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106646 ((S)-3-(4-Hydroxy-phenyl)-2-((R)-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor Beta | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558579 (CHEMBL4787989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA binding domain fused full-length chimeric estrogen receptor beta (unknown origin) by FRET-based assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187243 (17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at full length human ERbeta receptor assessed as transcriptional activity incubated for 22 to 24 hrs by cell based luciferase report... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115670 BindingDB Entry DOI: 10.7270/Q2ZK5MCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558587 (CHEMBL4750844) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187954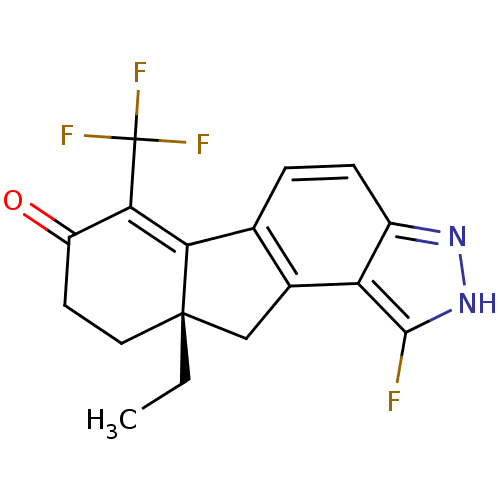 ((S)-9a-ethyl-1-fluoro-6-(trifluoromethyl)-8,9,9a,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human ERbeta transfected in HEK293 cells assessed as transactivation of alkaline phosphatase reporter gene | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluormone from GST-tagged ERbeta receptor LBD (unknown origin) measured after 60 mins by TR-FRET competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115670 BindingDB Entry DOI: 10.7270/Q2ZK5MCF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558592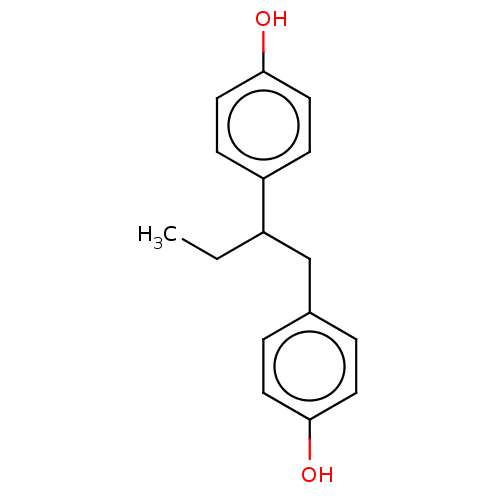 ((+/-)-Isobutestrol | Isobutesrol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50346462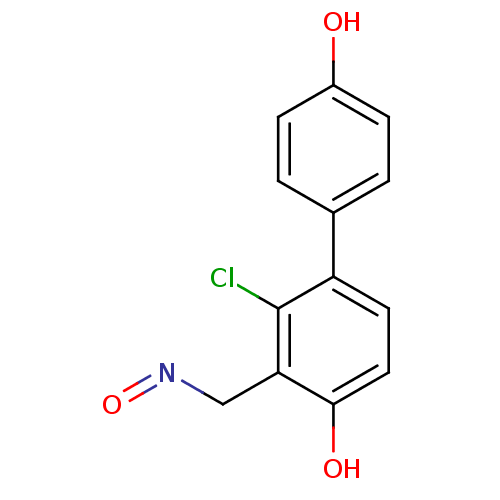 ((E)-2-chloro-4,4'-dihydroxybiphenyl-3-carbaldehyde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human full-length ERbeta receptor expressed in human HEC1 cells co-expressing (ERE)2-pS2-luc gene assessed as transcriptional act... | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of fluorescein-tagged estrogen binding to GST-tagged ERbeta (unknown origin) ligand binding domain after 1 hr by LanthaScreen TR-FRET assa... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assay | Bioorg Med Chem Lett 21: 5680-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.041 BindingDB Entry DOI: 10.7270/Q2JS9QTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50561302 (CHEMBL4782490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluormone from GST-tagged ERbeta receptor LBD (unknown origin) measured after 60 mins by TR-FRET competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115670 BindingDB Entry DOI: 10.7270/Q2ZK5MCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human ERbeta in human MCF7 cells assessed as otubain 2 endogenous genes activation after 24 hrs by qPCR | J Med Chem 58: 1184-94 (2015) Article DOI: 10.1021/jm501829f BindingDB Entry DOI: 10.7270/Q2XK8H8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173667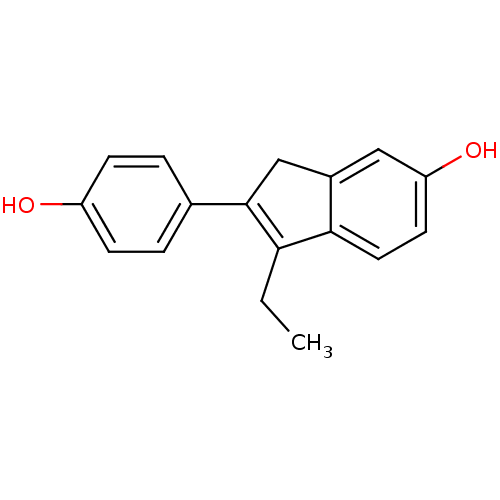 (1-Ethyl-2-(4-hydroxy-phenyl)-3H-inden-5-ol | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Transcriptional activation of estrogen receptor beta in U2OS cell using estrogen-regulated luciferase reporter gene plasmid upon incubation for 20-30... | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173661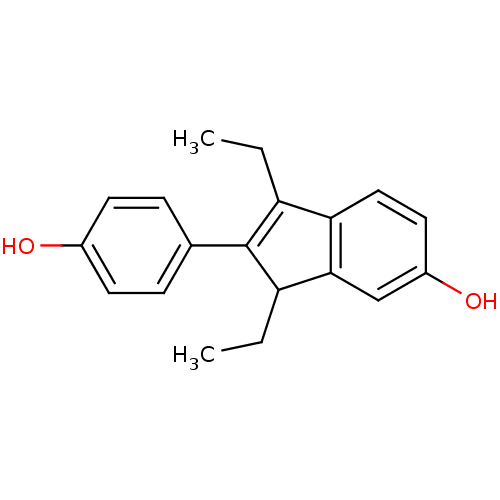 (1,3-Diethyl-2-(4-hydroxy-phenyl)-3H-inden-5-ol | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Transcriptional activation of estrogen receptor beta in U2OS cell using estrogen-regulated luciferase reporter gene plasmid upon incubation for 20-30... | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558583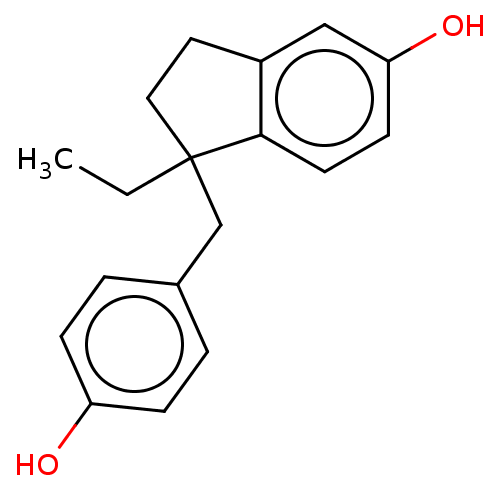 (CHEMBL4757650) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Agonist activity at ERbeta (unknown origin) expressed in human HeLa cells assessed as transcriptional activation measured after 24 hrs by luciferase ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115274 BindingDB Entry DOI: 10.7270/Q2XD157Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106643 (3-(4-Hydroxy-2-methyl-phenyl)-2-(4-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor Beta | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187243 (17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluormone from GST-tagged ERbeta receptor LBD (unknown origin) measured after 60 mins by TR-FRET competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115670 BindingDB Entry DOI: 10.7270/Q2ZK5MCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in human HEC1 cells assessed as transcriptional activation after 24 hrs by ERE-luciferase reporter gene tr... | J Med Chem 58: 1184-94 (2015) Article DOI: 10.1021/jm501829f BindingDB Entry DOI: 10.7270/Q2XK8H8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187943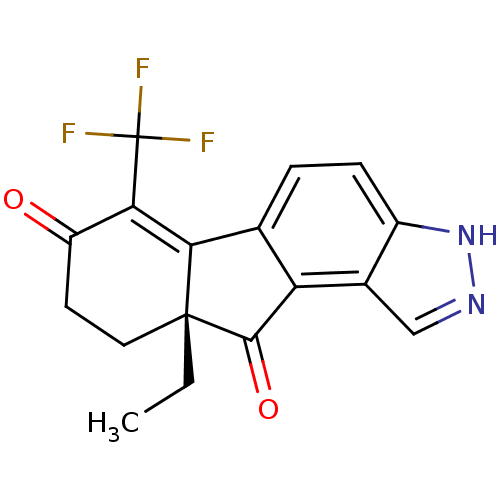 ((R)-9a-ethyl-6-(trifluoromethyl)-9,9a-dihydroinden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human ERbeta transfected in HEK293 cells assessed as transactivation of alkaline phosphatase reporter gene | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50189078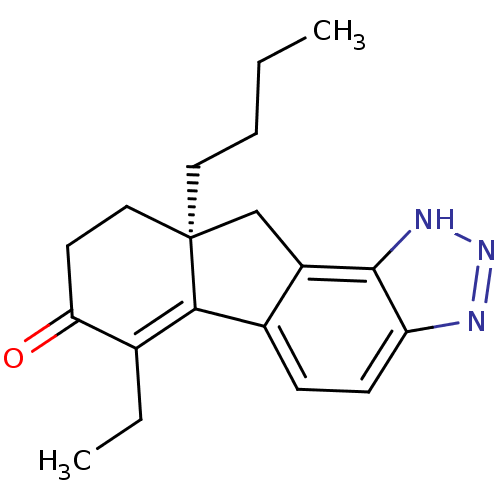 ((S)(-)-9a-Butyl-6-ethyl-8,9,9a,10-tetrahydro-3H-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant ERbeta expressed in HEK293 cells by alkaline phosphatase reporter gene transactivation assay | Bioorg Med Chem Lett 16: 4652-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.103 BindingDB Entry DOI: 10.7270/Q2MP52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERbeta expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106644 (2,3-Bis-(4-hydroxy-phenyl)-succinonitrile | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor Beta | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106644 (2,3-Bis-(4-hydroxy-phenyl)-succinonitrile | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor Beta | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558584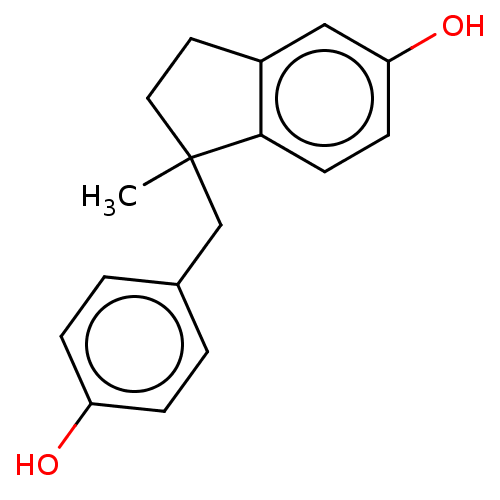 (CHEMBL4790777) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.458 | n/a | n/a | n/a | n/a |
Charles University in Prague Curated by ChEMBL | Assay Description Agonist activity at ERbeta-LBD in human U2OS cells transfected with Gal4-DBD assessed as increase of transactivation activity after 18 hrs by lucifer... | J Med Chem 53: 6947-53 (2010) Article DOI: 10.1021/jm100563h BindingDB Entry DOI: 10.7270/Q2PC33M2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 794 total ) | Next | Last >> |