

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM205429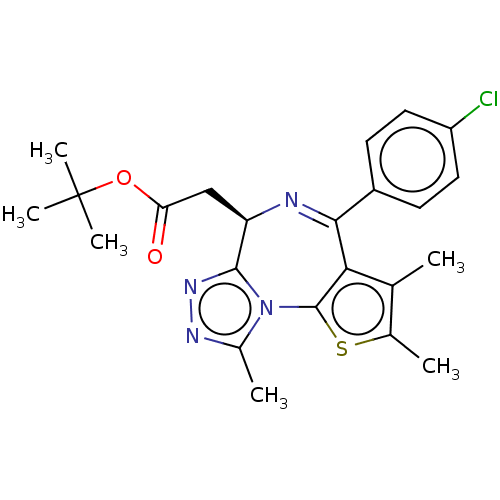 ( (R)-JQ1 (3) | US10407441, Compound (R)-JQ1 | US10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM50092323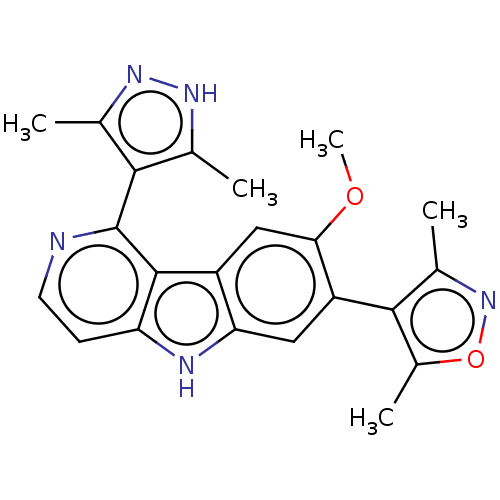 (CHEMBL3581656 | US9675697, Cpd. No. 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | <10 | <-10.9 | 93 | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313803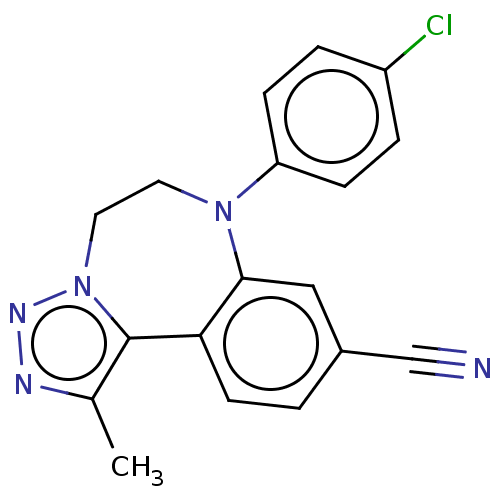 (US10167292, Example 47) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313802 (US10167292, Example 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313798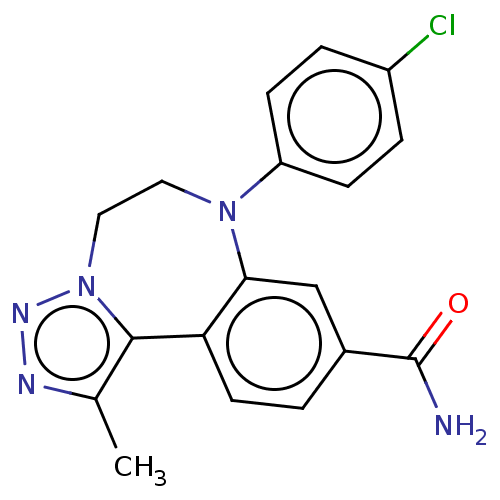 (US10167292, Example 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313805 (US10167292, Example 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313804 (US10167292, Example 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313771 (US10167292, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313807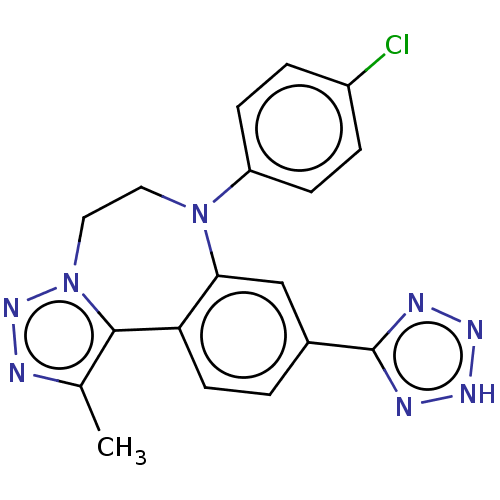 (US10167292, Example 51B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313773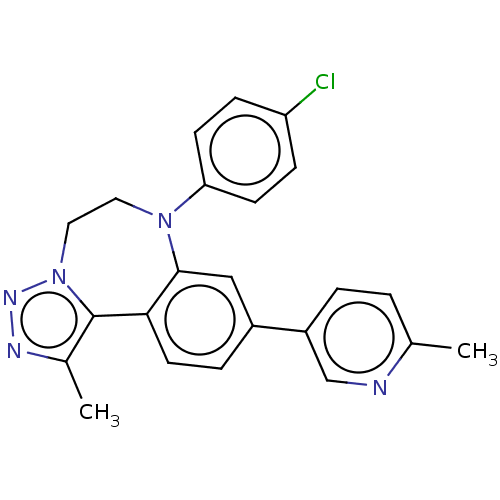 (US10167292, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313774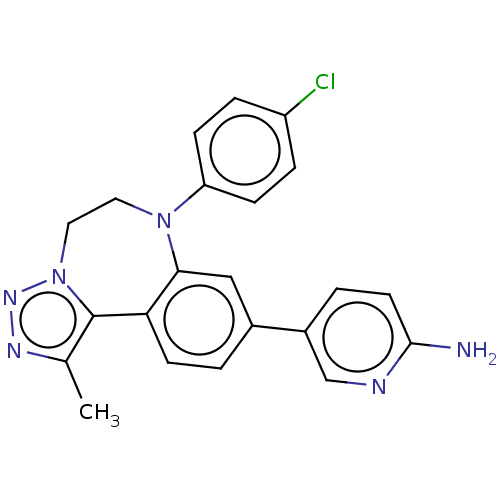 (US10167292, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313775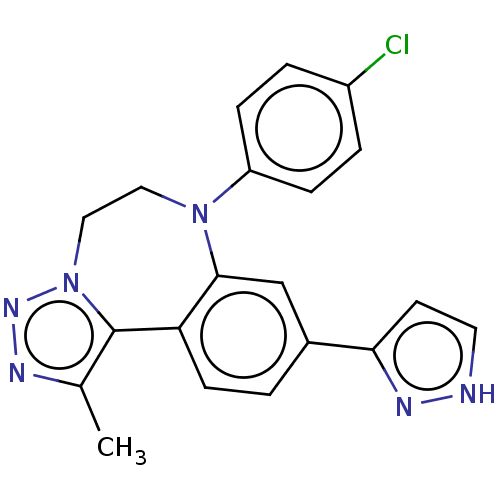 (US10167292, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313776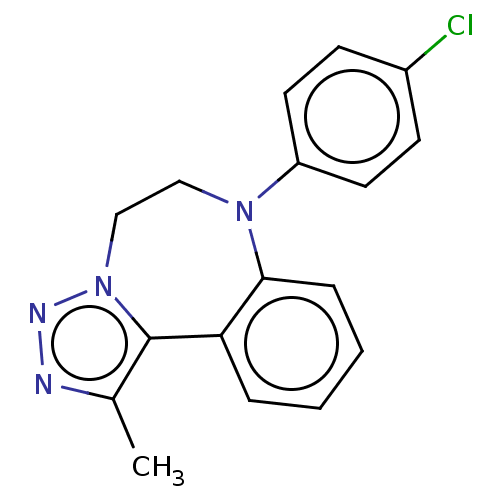 (US10167292, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313777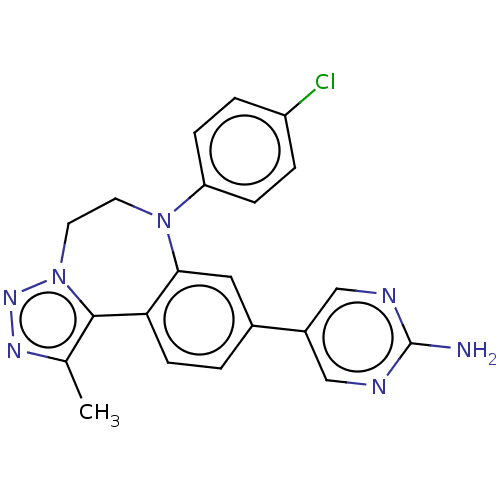 (US10167292, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313778 (US10167292, Example 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313779 (US10167292, Example 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313801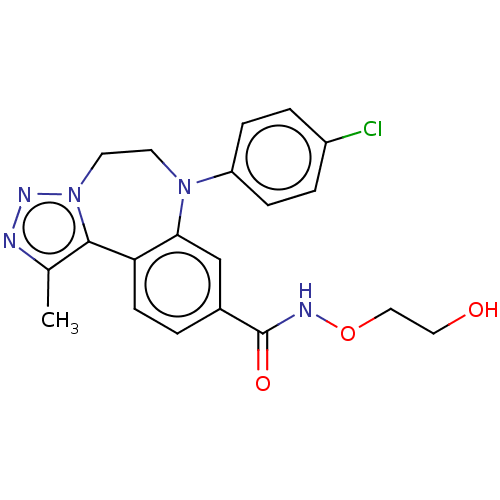 (US10167292, Example 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313781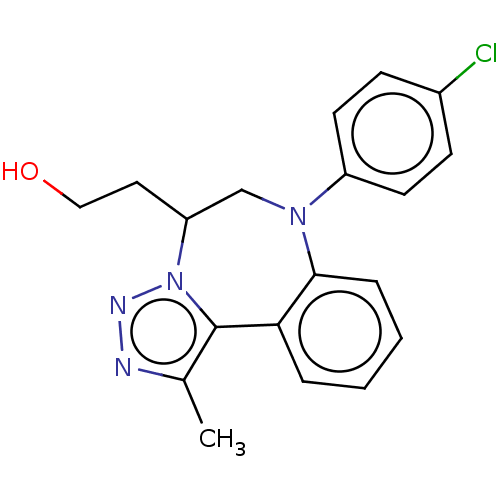 (US10167292, Example 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313782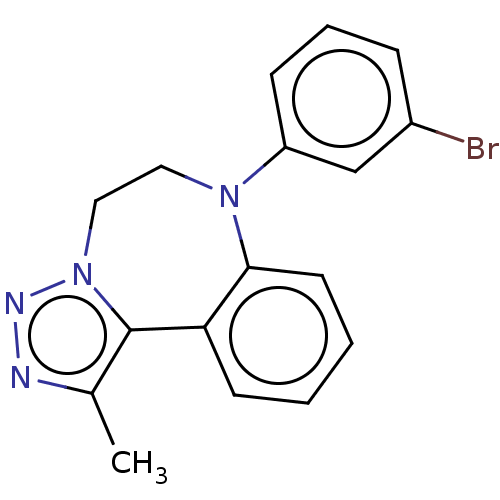 (US10167292, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313783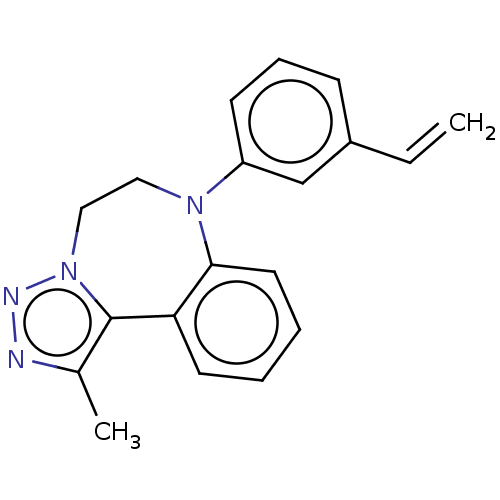 (US10167292, Example 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313784 (US10167292, Example 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313800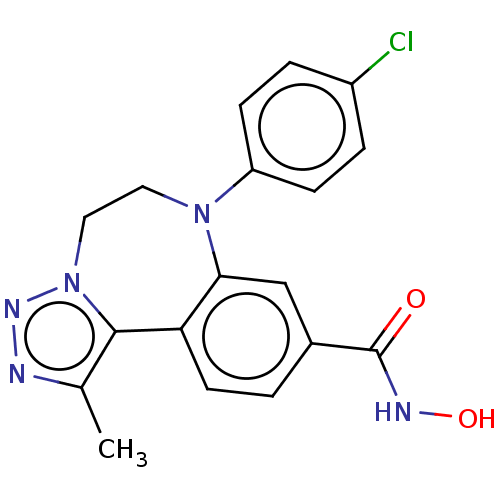 (US10167292, Example 44) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313799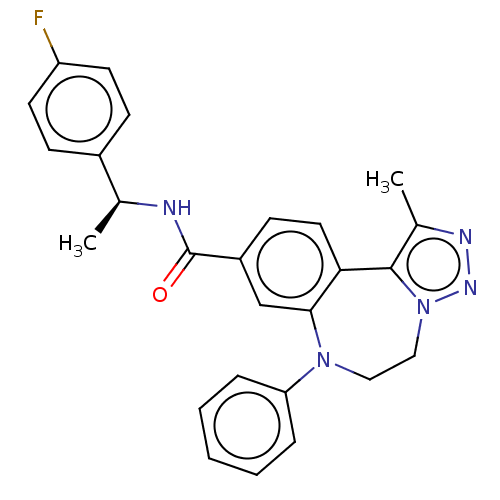 (US10167292, Example 43) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313787 (US10167292, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313788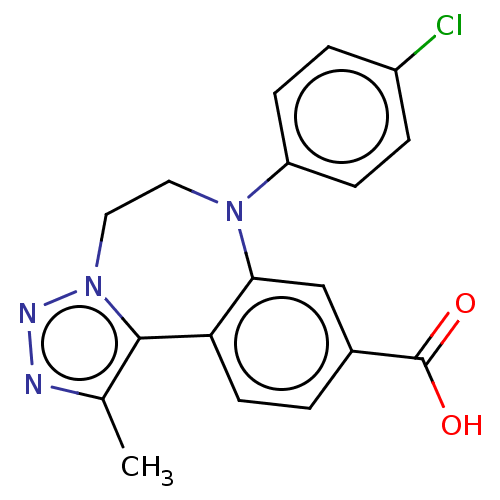 (US10167292, Example 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313789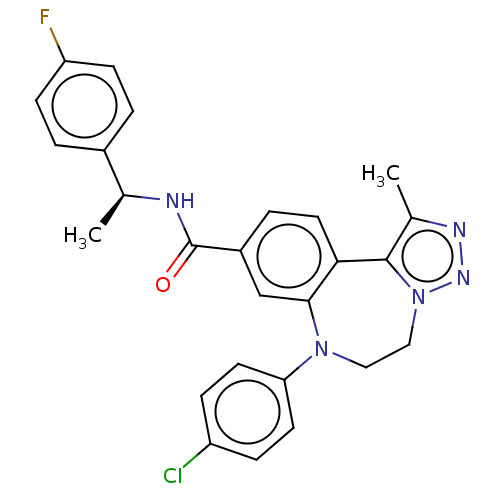 (US10167292, Example 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313790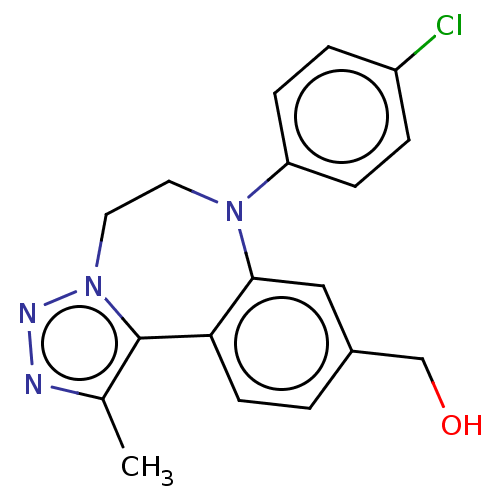 (US10167292, Example 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313791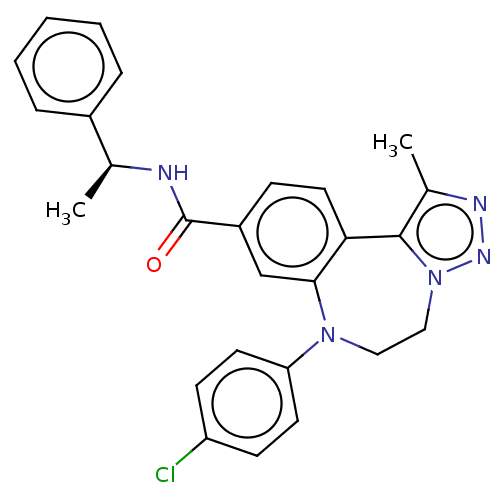 (US10167292, Example 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313792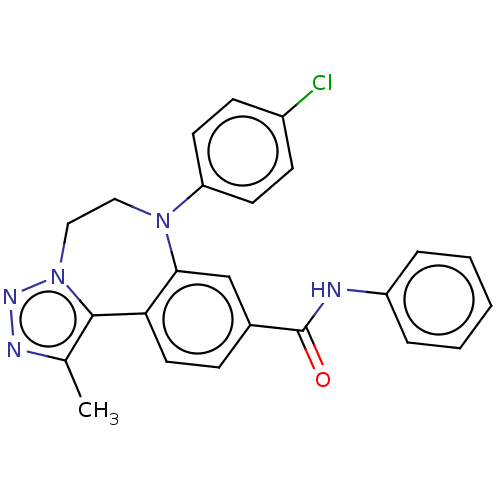 (US10167292, Example 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313793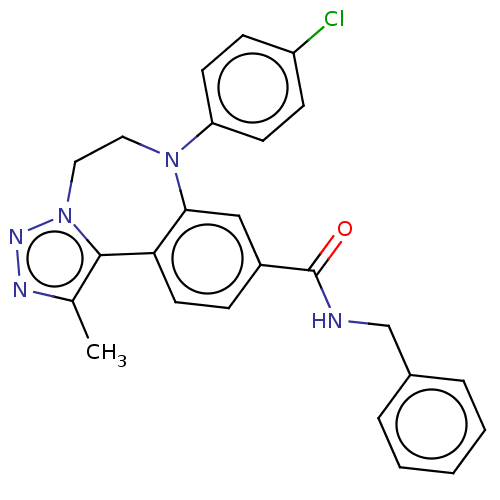 (US10167292, Example 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313794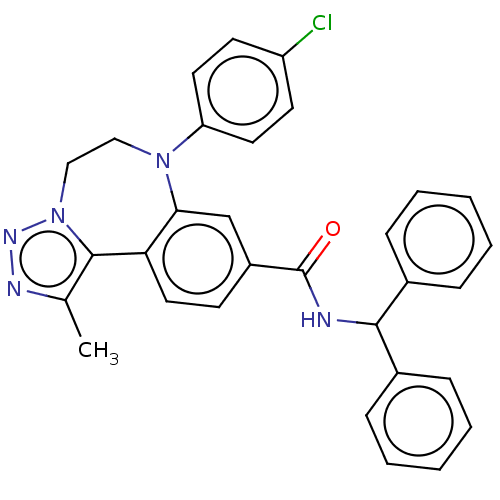 (US10167292, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313795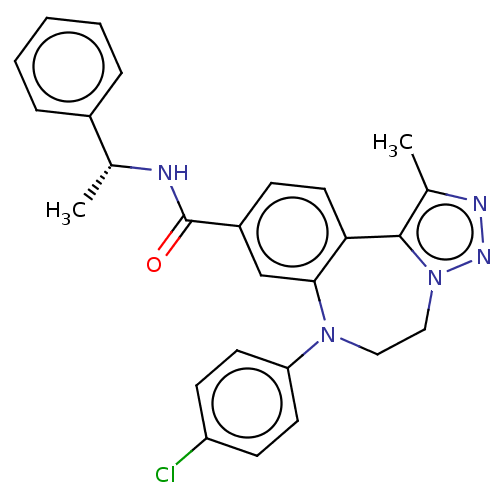 (US10167292, Example 39) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313796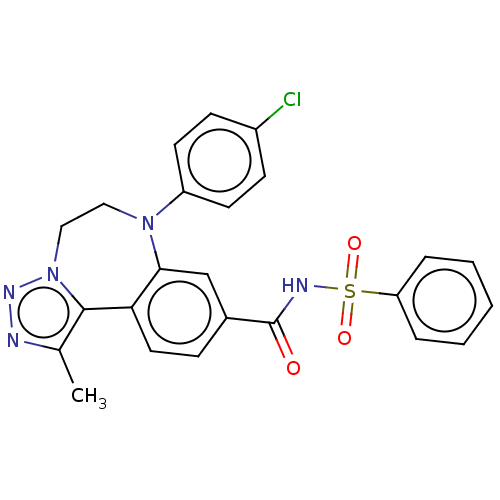 (US10167292, Example 40) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313797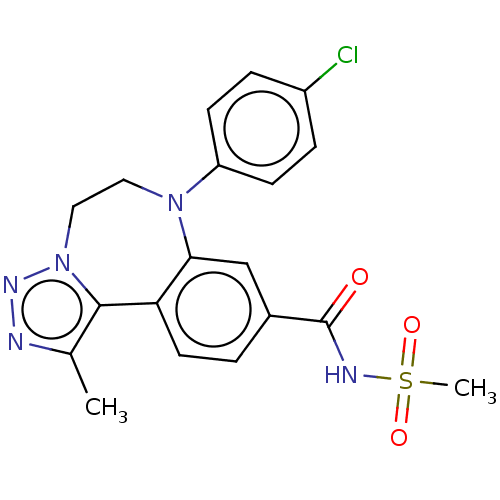 (US10167292, Example 41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM179460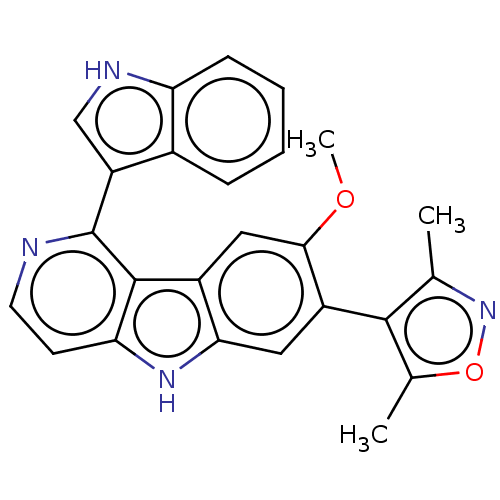 (US9675697, Cpd. No. 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <10 | <-10.9 | 142 | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM50103503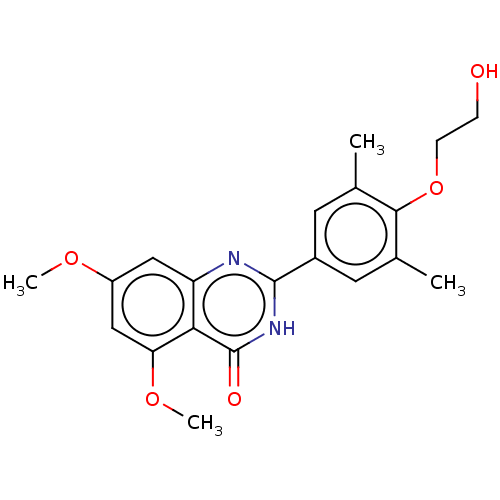 (CHEMBL2393130 | US11117865, Compound RVX-208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM179457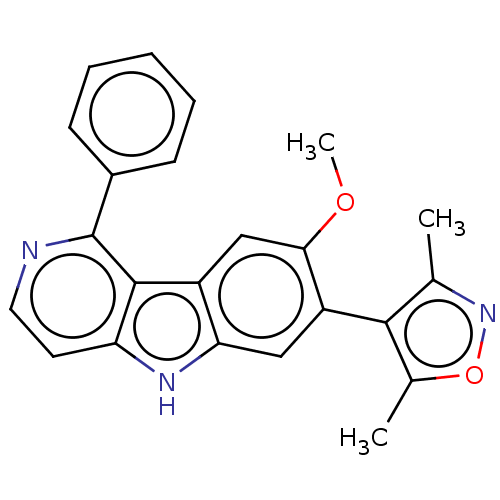 (US9675697, Cpd. No. 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 35 | -10.2 | 255 | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM50092318 (CHEMBL3581657 | US9675697, Cpd. No. 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 42.3 | -10.1 | 286 | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM179458 (US9675697, Cpd. No. 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 57 | -9.88 | 349 | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description The FAM labeled fluorescent probe (BRD-1F) was synthesized based on a known small-molecule BET bromodomain inhibitor. Kd values of BRD-1F to these th... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313785 (US10167292, Example 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313780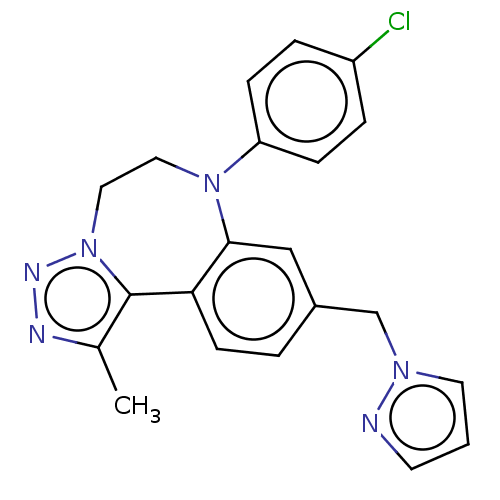 (US10167292, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313806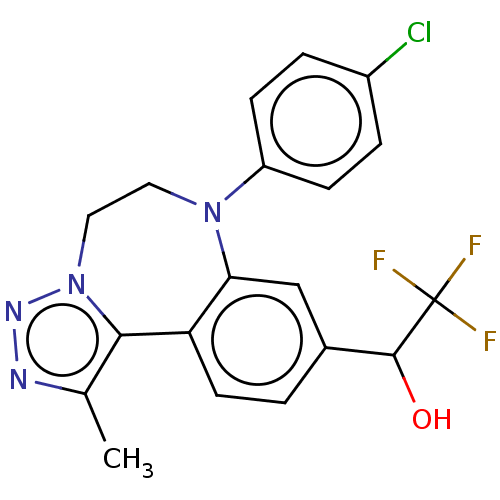 (US10167292, Example 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313786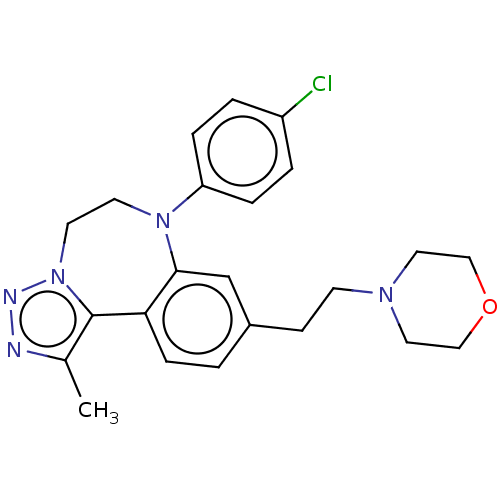 (US10167292, Example 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM50527819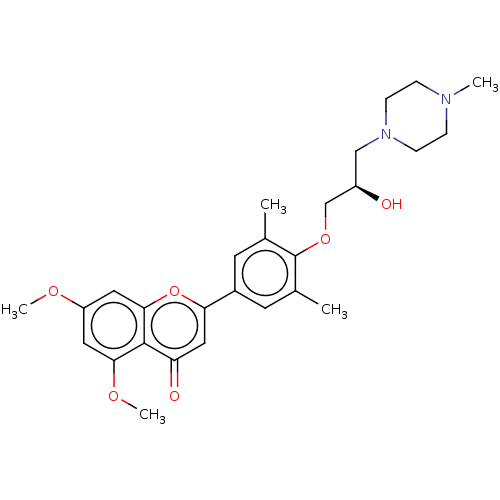 (CHEMBL4557050 | US11117865, Compound ZL0516) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 914 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM50527819 (CHEMBL4557050 | US11117865, Compound ZL0516) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 914 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM313772 (US10167292, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM518731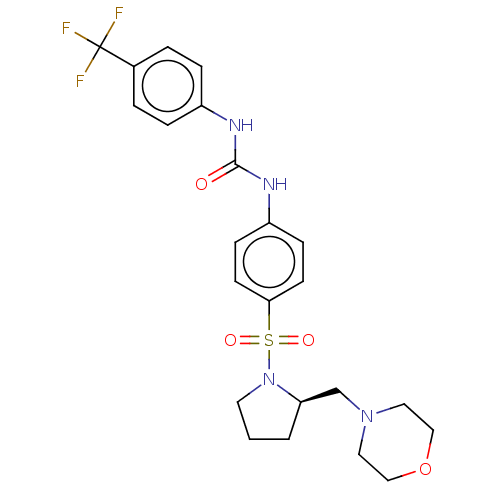 (US11117865, Compound ZL0590) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM518731 (US11117865, Compound ZL0590) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM518799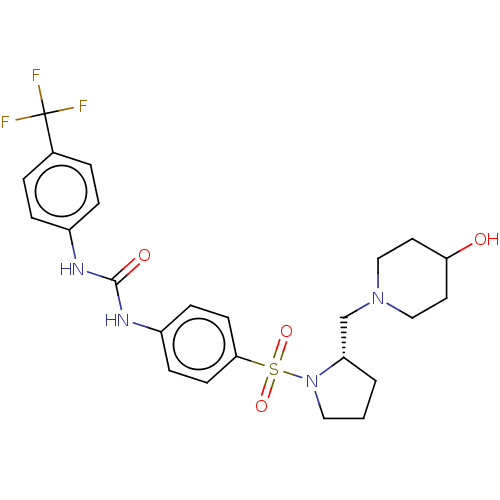 (US11117865, Compound ZL0591) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [364-436] (Homo sapiens (Human)) | BDBM518799 (US11117865, Compound ZL0591) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |