 Found 265 hits of ec50 data for polymerid = 3972,50000055
Found 265 hits of ec50 data for polymerid = 3972,50000055 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032223

(4-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1csc(c1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O2S/c1-14(9-16-11-20(21(24)25)26-13-16)17-12-19-18(10-15(17)2)22(3,4)7-8-23(19,5)6/h9-13H,7-8H2,1-6H3,(H,24,25)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032224

(3-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1cccc(c1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H30O2/c1-16(12-18-8-7-9-19(14-18)23(26)27)20-15-22-21(13-17(20)2)24(3,4)10-11-25(22,5)6/h7-9,12-15H,10-11H2,1-6H3,(H,26,27)/b16-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032218
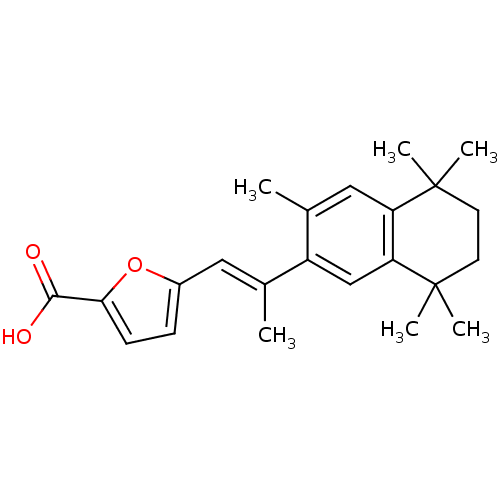
(5-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ccc(o1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O3/c1-14(11-16-7-8-20(26-16)21(24)25)17-13-19-18(12-15(17)2)22(3,4)9-10-23(19,5)6/h7-8,11-13H,9-10H2,1-6H3,(H,24,25)/b14-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032225
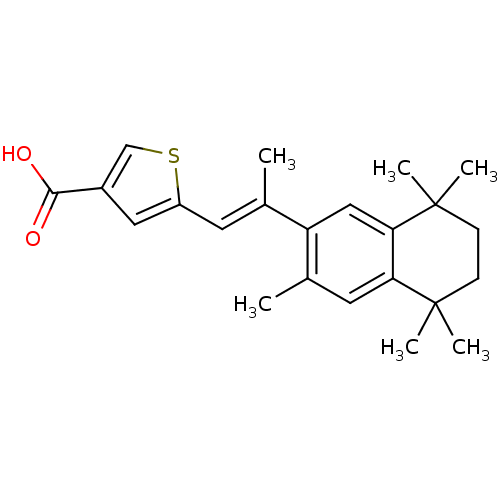
(5-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1cc(cs1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O2S/c1-14(9-17-11-16(13-26-17)21(24)25)18-12-20-19(10-15(18)2)22(3,4)7-8-23(20,5)6/h9-13H,7-8H2,1-6H3,(H,24,25)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032220

(5-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ccc(s1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O2S/c1-14(11-16-7-8-20(26-16)21(24)25)17-13-19-18(12-15(17)2)22(3,4)9-10-23(19,5)6/h7-8,11-13H,9-10H2,1-6H3,(H,24,25)/b14-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219
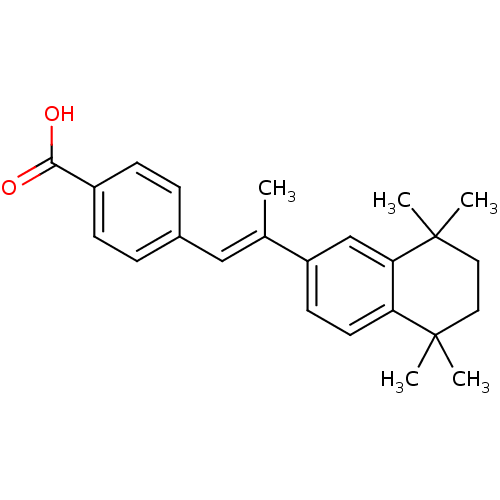
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human RARalpha expressed in HEK293 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052414
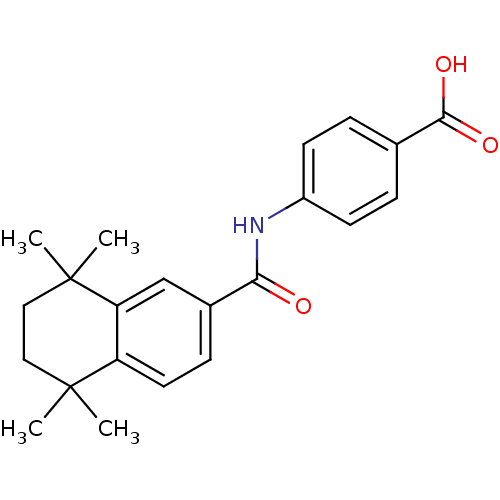
(4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-15(7-10-17(18)21)19(24)23-16-8-5-14(6-9-16)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity at human RARalpha ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ... |
Bioorg Med Chem Lett 19: 489-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.040
BindingDB Entry DOI: 10.7270/Q2GF0TC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50265920

(4-((5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24O2/c1-22(2)13-14-23(3,4)20-15-17(9-12-19(20)22)6-5-16-7-10-18(11-8-16)21(24)25/h7-12,15H,13-14H2,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity at human RARalpha ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ... |
Bioorg Med Chem Lett 19: 489-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.040
BindingDB Entry DOI: 10.7270/Q2GF0TC9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892
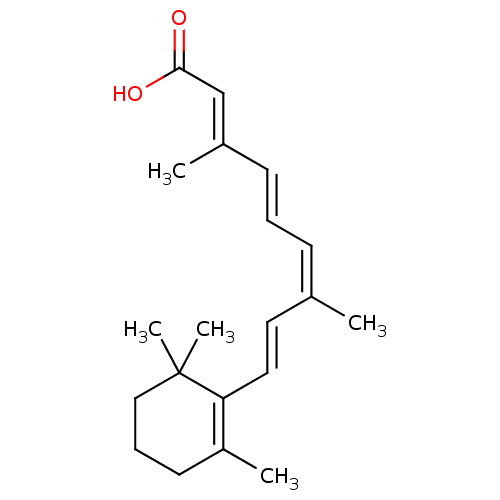
(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00641
BindingDB Entry DOI: 10.7270/Q2K35ZR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883
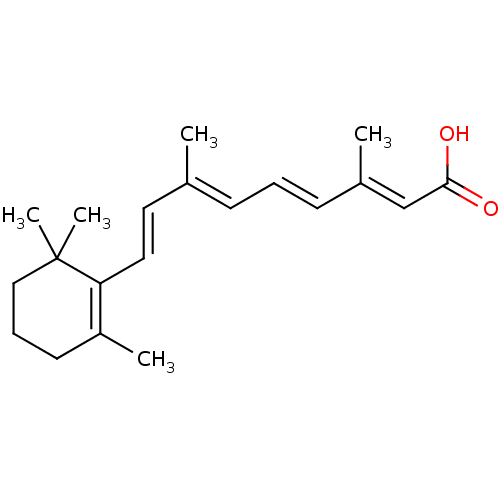
(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Transactivation of human Gal4-DBD-fused RARalpha-LBD expressed in HEK293T cells after 16 to 24 hrs by FRET based beta-lactamase reporter gene assay |
Bioorg Med Chem 26: 798-814 (2018)
Article DOI: 10.1016/j.bmc.2017.12.015
BindingDB Entry DOI: 10.7270/Q2WH2SKR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM323588
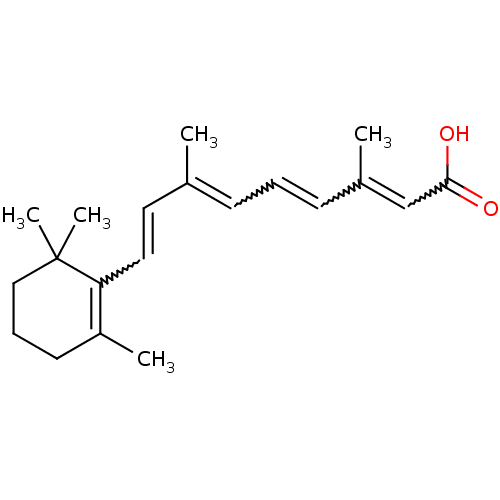
(Retinoic Acid | US10188615, at-RA | US10752616, Co...)Show SMILES CC(C=CC1=C(C)CCCC1(C)C)=CC=CC(C)=CC(O)=O |w:3.3,13.14,15.16,18.19,c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052414

(4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-15(7-10-17(18)21)19(24)23-16-8-5-14(6-9-16)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Effective concentration for retinoic acid receptor RAR gamma transcriptional activation |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032667
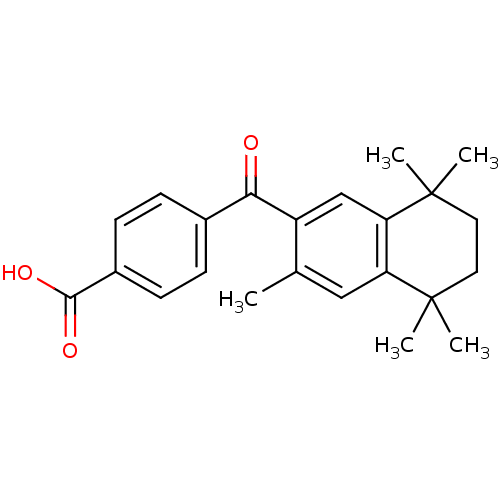
(4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1C(=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H26O3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24)15-6-8-16(9-7-15)21(25)26/h6-9,12-13H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressiing Retinoic acid receptor RAR alpha |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052414

(4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-15(7-10-17(18)21)19(24)23-16-8-5-14(6-9-16)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at RARalpha by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052589
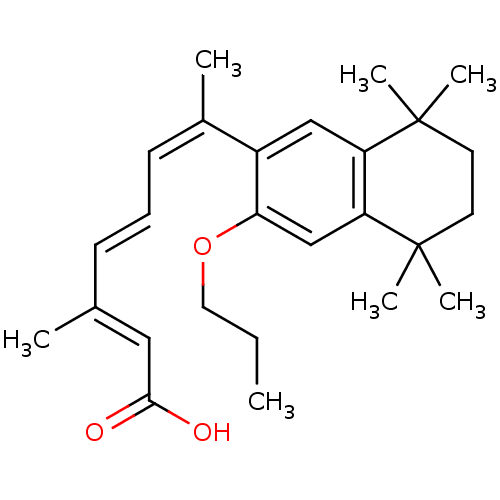
((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...)Show SMILES CCCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H36O3/c1-8-14-29-23-17-22-21(25(4,5)12-13-26(22,6)7)16-20(23)19(3)11-9-10-18(2)15-24(27)28/h9-11,15-17H,8,12-14H2,1-7H3,(H,27,28)/b10-9+,18-15+,19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation of retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 5: 523-527 (1995)
Article DOI: 10.1016/0960-894X(95)00065-2
BindingDB Entry DOI: 10.7270/Q20G3K4D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 42: 4961-9 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PX3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM323587
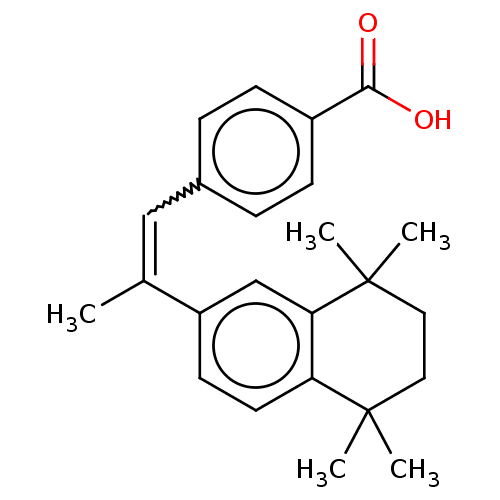
(4-[(E)-2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2...)Show SMILES CC(=Cc1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C |w:2.2| Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
ACUCELA INC.
US Patent
| Assay Description
Retinoid nuclear receptor activity is associated with transduction of the non-visual physiologic, pharmacologic, and toxicologic retinoid signals tha... |
US Patent US10188615 (2019)
BindingDB Entry DOI: 10.7270/Q2NP26HC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressiing Retinoic acid receptor RAR alpha |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB
Curated by ChEMBL
| Assay Description
Activity at RARalpha expressed in mouse NIH3T3 cells by R-SAT assay |
J Med Chem 52: 1540-5 (2009)
Article DOI: 10.1021/jm801532e
BindingDB Entry DOI: 10.7270/Q2K938R9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against retinoid X receptor using 5 nM of [3H]-9-cis-RA as a radioligand in baculovirus expressed receptor |
Bioorg Med Chem Lett 6: 213-218 (1996)
Article DOI: 10.1016/0960-894X(95)00588-K
BindingDB Entry DOI: 10.7270/Q2BC3ZHZ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing Retinoic acid receptor RAR alpha |
J Med Chem 38: 4764-7 (1996)
BindingDB Entry DOI: 10.7270/Q22N5194 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50120066

(2-Fluoro-4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydr...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C22H24FNO3/c1-21(2)9-10-22(3,4)17-11-13(5-8-16(17)21)19(25)24-14-6-7-15(20(26)27)18(23)12-14/h5-8,11-12H,9-10H2,1-4H3,(H,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor RAR gamma |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50460102

(CHEMBL4224900)Show SMILES Cc1sc(nc1-c1ccc2c(c1)C(C)(C)CCC2(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27NO2S/c1-15-21(26-22(29-15)16-6-8-17(9-7-16)23(27)28)18-10-11-19-20(14-18)25(4,5)13-12-24(19,2)3/h6-11,14H,12-13H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
Helwan University
Curated by ChEMBL
| Assay Description
Transactivation of GST-tagged RARalpha LBD (unknown origin) assessed as fluorescein-labelled coactivator peptide recruitment measured after 4 hrs by ... |
Bioorg Med Chem 26: 1560-1572 (2018)
Article DOI: 10.1016/j.bmc.2018.02.002
BindingDB Entry DOI: 10.7270/Q2WQ06F7 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM458284
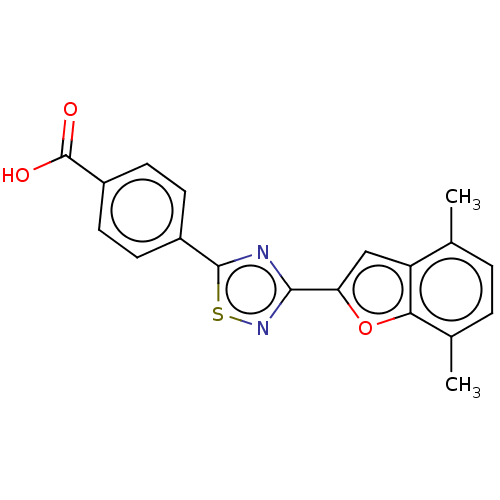
(US10752616, Code No. BHBA-042)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nsc(n1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O3S/c1-10-3-4-11(2)16-14(10)9-15(24-16)17-20-18(25-21-17)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50282692

(4-[(E)-2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C23H26O2/c1-22(2)13-14-23(3,4)20-15-17(9-12-19(20)22)6-5-16-7-10-18(11-8-16)21(24)25/h5-12,15H,13-14H2,1-4H3,(H,24,25)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50457542
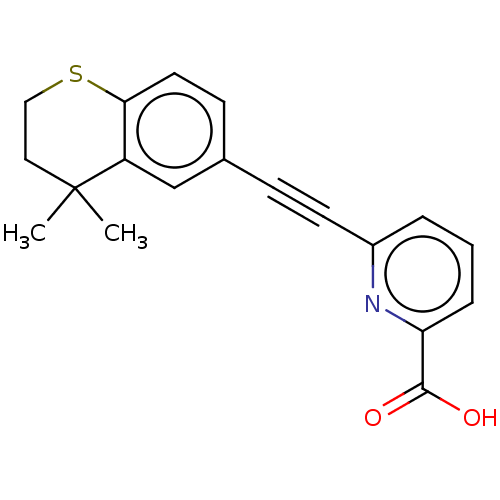
(CHEMBL4212472)Show InChI InChI=1S/C19H17NO2S/c1-19(2)10-11-23-17-9-7-13(12-15(17)19)6-8-14-4-3-5-16(20-14)18(21)22/h3-5,7,9,12H,10-11H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARalpha (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM458233
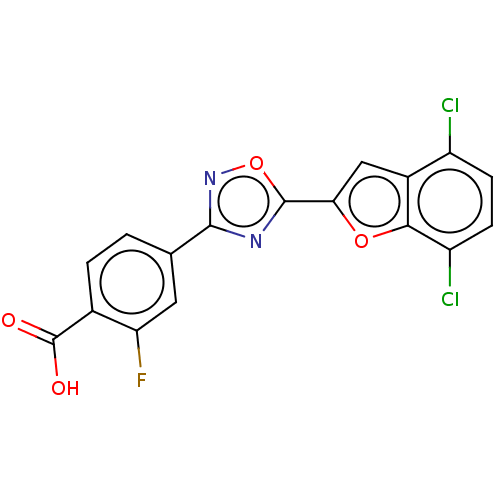
(US10752616, Code No. BHBA-014)Show SMILES OC(=O)c1ccc(cc1F)-c1noc(n1)-c1cc2c(Cl)ccc(Cl)c2o1 Show InChI InChI=1S/C17H7Cl2FN2O4/c18-10-3-4-11(19)14-9(10)6-13(25-14)16-21-15(22-26-16)7-1-2-8(17(23)24)12(20)5-7/h1-6H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052590
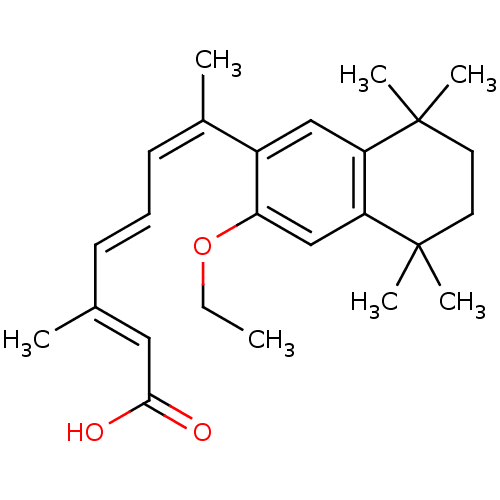
((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES CCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H34O3/c1-8-28-22-16-21-20(24(4,5)12-13-25(21,6)7)15-19(22)18(3)11-9-10-17(2)14-23(26)27/h9-11,14-16H,8,12-13H2,1-7H3,(H,26,27)/b10-9+,17-14+,18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50282691
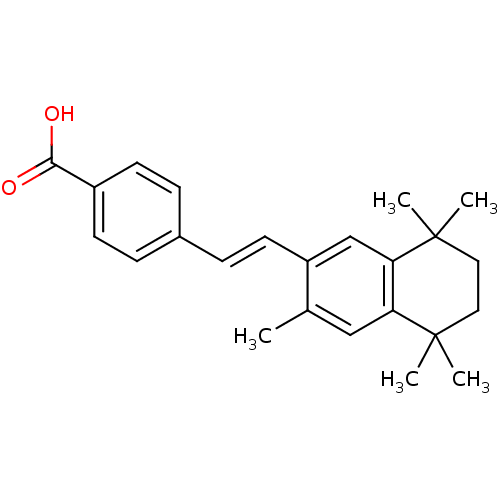
(4-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES Cc1cc2c(cc1\C=C\c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16-14-20-21(24(4,5)13-12-23(20,2)3)15-19(16)11-8-17-6-9-18(10-7-17)22(25)26/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50413270
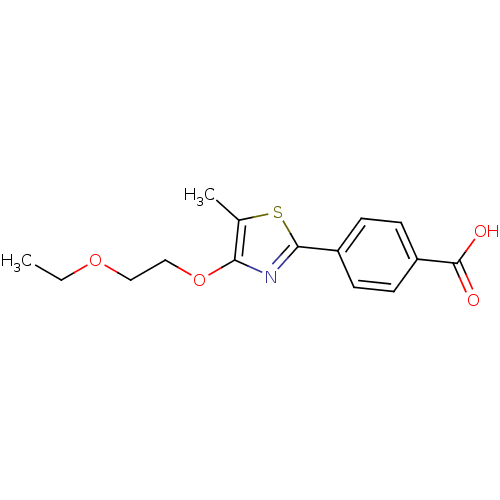
(CHEMBL512879)Show InChI InChI=1S/C15H17NO4S/c1-3-19-8-9-20-13-10(2)21-14(16-13)11-4-6-12(7-5-11)15(17)18/h4-7H,3,8-9H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Helwan University
Curated by ChEMBL
| Assay Description
Transactivation of GST-tagged RARalpha LBD (unknown origin) assessed as fluorescein-labelled coactivator peptide recruitment measured after 4 hrs by ... |
Bioorg Med Chem 26: 1560-1572 (2018)
Article DOI: 10.1016/j.bmc.2018.02.002
BindingDB Entry DOI: 10.7270/Q2WQ06F7 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Helwan University
Curated by ChEMBL
| Assay Description
Transactivation of GST-tagged RARalpha LBD (unknown origin) assessed as fluorescein-labelled coactivator peptide recruitment measured after 4 hrs by ... |
Bioorg Med Chem 26: 1560-1572 (2018)
Article DOI: 10.1016/j.bmc.2018.02.002
BindingDB Entry DOI: 10.7270/Q2WQ06F7 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50526312

(CHEMBL4449668 | US10752616, Code No. BHBA-007)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C19H13FN2O4/c1-9-3-4-10(2)16-13(9)8-15(25-16)18-21-17(22-26-18)11-5-6-12(19(23)24)14(20)7-11/h3-8H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity at human RARalpha ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ... |
Bioorg Med Chem Lett 19: 489-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.040
BindingDB Entry DOI: 10.7270/Q2GF0TC9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM458145
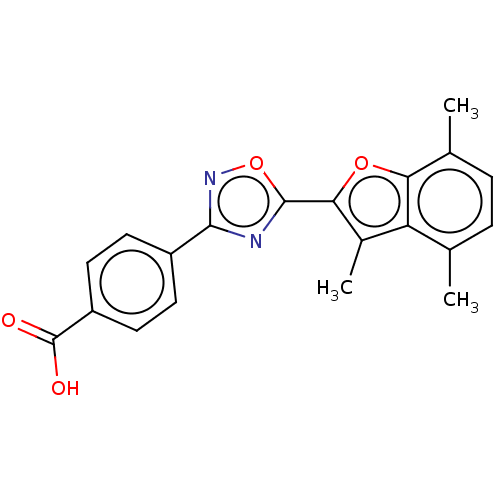
(US10752616, Code No. BHBA-008)Show SMILES Cc1c(oc2c(C)ccc(C)c12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H16N2O4/c1-10-4-5-11(2)16-15(10)12(3)17(25-16)19-21-18(22-26-19)13-6-8-14(9-7-13)20(23)24/h4-9H,1-3H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290184
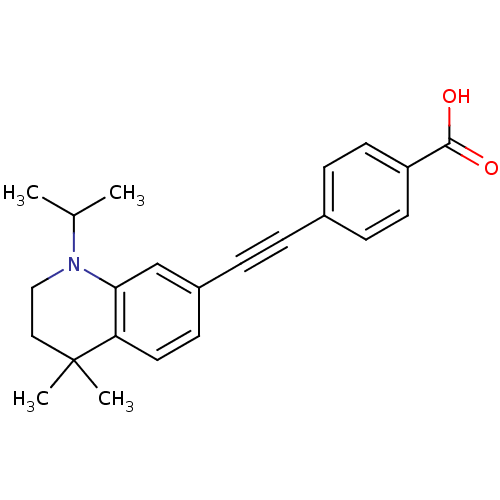
(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-12-9-18(15-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor alpha; Not active |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM458251

(US10752616, Code No. BHBA-016)Show SMILES Cc1ccc(Cl)c2cc(oc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H11ClN2O4/c1-9-2-7-13(19)12-8-14(24-15(9)12)17-20-16(21-25-17)10-3-5-11(6-4-10)18(22)23/h2-8H,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM458280
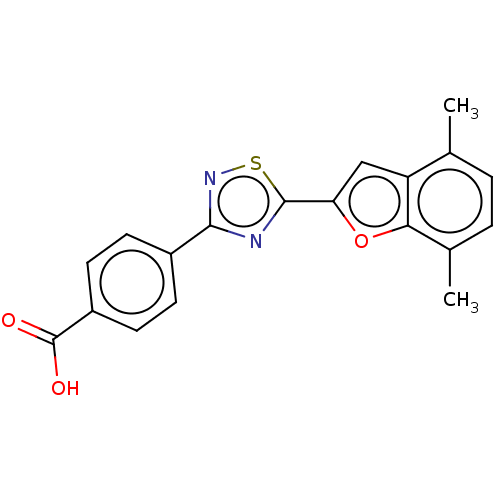
(US10752616, Code No. BHBA-038)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(ns1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O3S/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50526315
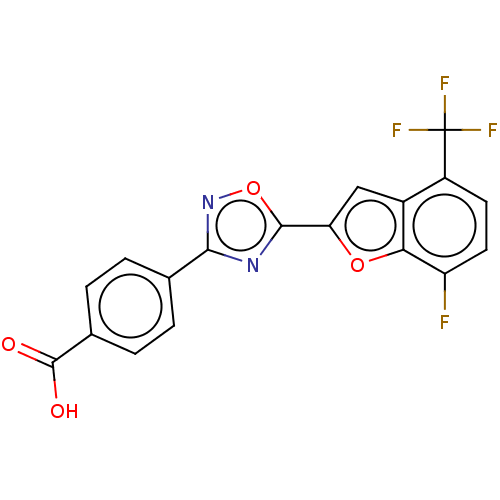
(CHEMBL4439399 | US10752616, Code No. BHBA-019)Show SMILES OC(=O)c1ccc(cc1)-c1noc(n1)-c1cc2c(ccc(F)c2o1)C(F)(F)F Show InChI InChI=1S/C18H8F4N2O4/c19-12-6-5-11(18(20,21)22)10-7-13(27-14(10)12)16-23-15(24-28-16)8-1-3-9(4-2-8)17(25)26/h1-7H,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
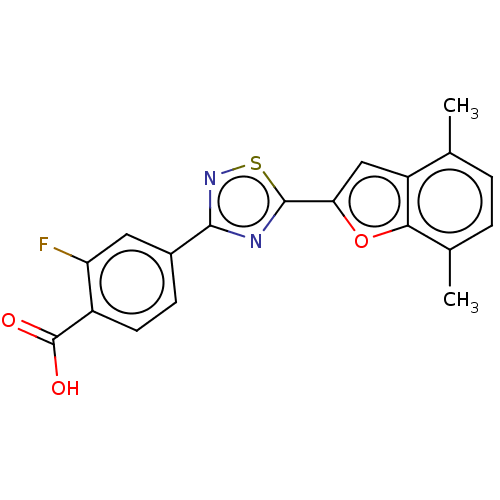
(Homo sapiens (Human)) | BDBM458283
(US10752616, Code No. BHBA-041)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(ns1)-c1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C19H13FN2O3S/c1-9-3-4-10(2)16-13(9)8-15(25-16)18-21-17(22-26-18)11-5-6-12(19(23)24)14(20)7-11/h3-8H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation of retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 5: 523-527 (1995)
Article DOI: 10.1016/0960-894X(95)00065-2
BindingDB Entry DOI: 10.7270/Q20G3K4D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048280

(6-(3-(1-Adamantyl)-4-methoxyphenyl)-2-naphthoic ac...)Show SMILES COc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc2cc(ccc2c1)C(O)=O |TLB:7:8:11:15.14.13,THB:9:10:13:17.8.16,9:8:11.10.15:13,16:8:11:15.14.13,16:14:11:17.9.8| Show InChI InChI=1S/C28H28O3/c1-31-26-7-6-23(21-2-3-22-12-24(27(29)30)5-4-20(22)11-21)13-25(26)28-14-17-8-18(15-28)10-19(9-17)16-28/h2-7,11-13,17-19H,8-10,14-16H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARalpha (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Relative activity against Retinoic acid receptor RAR alpha compared to ATRA |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM458240

(US10752616, Code No. BHBA-015)Show SMILES Cc1ccc(Cl)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H11ClN2O4/c1-9-2-7-13(19)15-12(9)8-14(24-15)17-20-16(21-25-17)10-3-5-11(6-4-10)18(22)23/h2-8H,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50045276
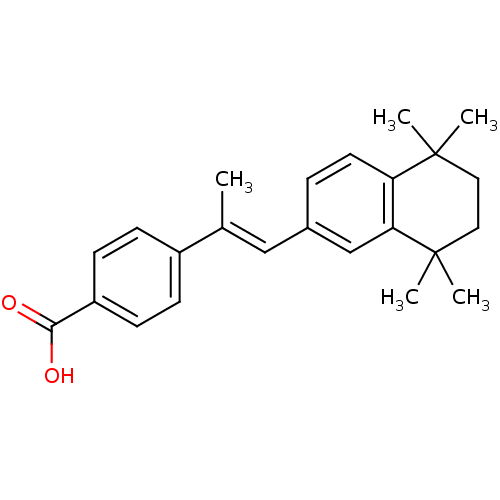
(4-[(E)-1-Methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tet...)Show SMILES C\C(=C/c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H28O2/c1-16(18-7-9-19(10-8-18)22(25)26)14-17-6-11-20-21(15-17)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR alpha receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data