 Found 44 hits of ic50 data for polymerid = 3972,50000055
Found 44 hits of ic50 data for polymerid = 3972,50000055 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084833

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27NO2/c1-24(2)13-14-25(3,4)20-15-18(9-10-19(20)24)22-12-11-21(26-22)16-5-7-17(8-6-16)23(27)28/h5-12,15,26H,13-14H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor alpha |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075879
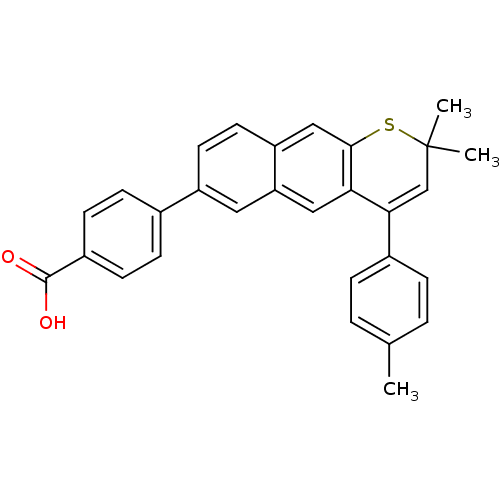
(4-(2,2-Dimethyl-4-p-tolyl-2H-1-thia-anthracen-6-yl...)Show SMILES Cc1ccc(cc1)C1=CC(C)(C)Sc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C29H24O2S/c1-18-4-6-20(7-5-18)26-17-29(2,3)32-27-16-23-13-12-22(14-24(23)15-25(26)27)19-8-10-21(11-9-19)28(30)31/h4-17H,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonistic activity against RAR gamma in transcriptional activation assay with 3.2 nM TTNPB |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084835

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25N3O2/c1-22(2)11-12-23(3,4)20-19(22)24-13-18(26-20)17-10-9-16(25-17)14-5-7-15(8-6-14)21(27)28/h5-10,13,25H,11-12H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084832
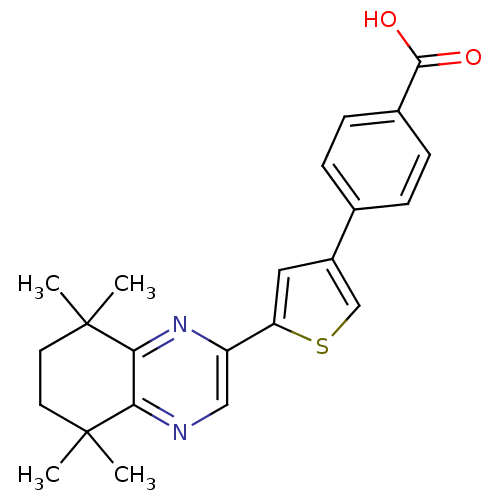
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075878
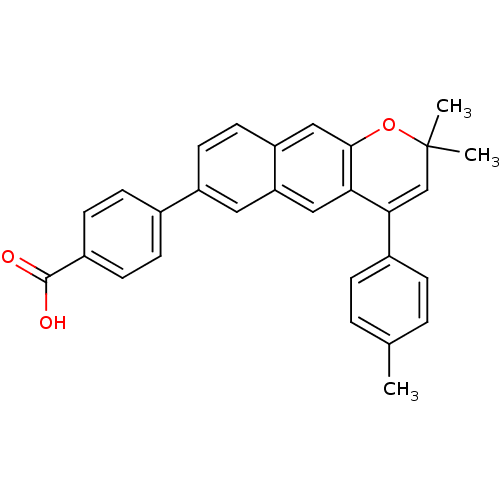
(4-(2,2-Dimethyl-4-p-tolyl-2H-benzo[g]chromen-7-yl)...)Show SMILES Cc1ccc(cc1)C1=CC(C)(C)Oc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C29H24O3/c1-18-4-6-20(7-5-18)26-17-29(2,3)32-27-16-23-13-12-22(14-24(23)15-25(26)27)19-8-10-21(11-9-19)28(30)31/h4-17H,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]- RA to baculovirus expressed human RAR alpha |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075876
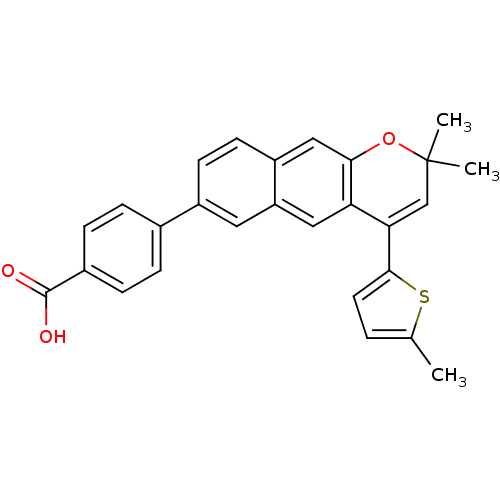
(4-[2,2-Dimethyl-4-(5-methyl-thiophen-2-yl)-2H-benz...)Show SMILES Cc1ccc(s1)C1=CC(C)(C)Oc2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:7| Show InChI InChI=1S/C27H22O3S/c1-16-4-11-25(31-16)23-15-27(2,3)30-24-14-20-10-9-19(12-21(20)13-22(23)24)17-5-7-18(8-6-17)26(28)29/h4-15H,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonistic activity against RAR alpha in transcriptional activation assay with 32 nM TTNPB |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50296049

((2E,4E)-2,4-dimethyl-5-(6,6,9,9-tetramethyl-4-oxo-...)Show SMILES C\C(\C=C(/C)C(O)=O)=C/c1coc2cc3c(cc2c1=O)C(C)(C)CCC3(C)C Show InChI InChI=1S/C24H28O4/c1-14(9-15(2)22(26)27)10-16-13-28-20-12-19-18(11-17(20)21(16)25)23(3,4)7-8-24(19,5)6/h9-13H,7-8H2,1-6H3,(H,26,27)/b14-10+,15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Displacement of radioligand from RARalpha receptor |
Bioorg Med Chem Lett 19: 4339-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.081
BindingDB Entry DOI: 10.7270/Q2N58MD3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084834
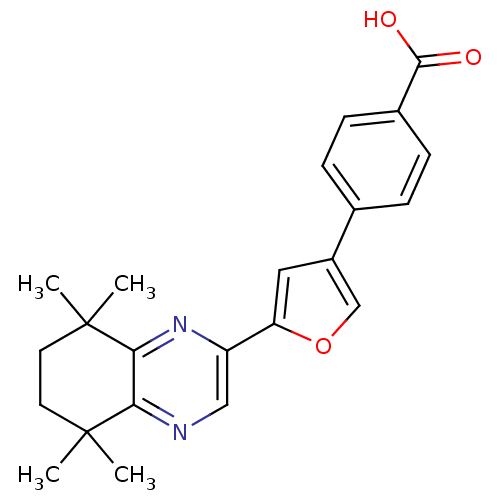
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(co1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075877
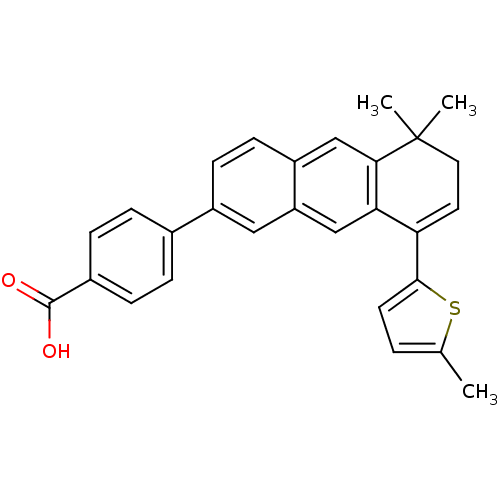
(4-[5,5-Dimethyl-8-(5-methyl-thiophen-2-yl)-5,6-dih...)Show SMILES Cc1ccc(s1)C1=CCC(C)(C)c2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:7| Show InChI InChI=1S/C28H24O2S/c1-17-4-11-26(31-17)23-12-13-28(2,3)25-16-21-10-9-20(14-22(21)15-24(23)25)18-5-7-19(8-6-18)27(29)30/h4-12,14-16H,13H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonistic activity against RAR beta in transcriptional activation assay with 10 nM TTNPB |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50075880

(4-(5,5-Dimethyl-8-p-tolyl-5,6-dihydro-anthracen-2-...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2cc3ccc(cc3cc12)-c1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C30H26O2/c1-19-4-6-21(7-5-19)26-14-15-30(2,3)28-18-24-13-12-23(16-25(24)17-27(26)28)20-8-10-22(11-9-20)29(31)32/h4-14,16-18H,15H2,1-3H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonistic activity against RAR alpha in transcriptional activation assay with 32 nM TTNPB |
Bioorg Med Chem Lett 9: 743-8 (1999)
BindingDB Entry DOI: 10.7270/Q26Q1WFP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50099474
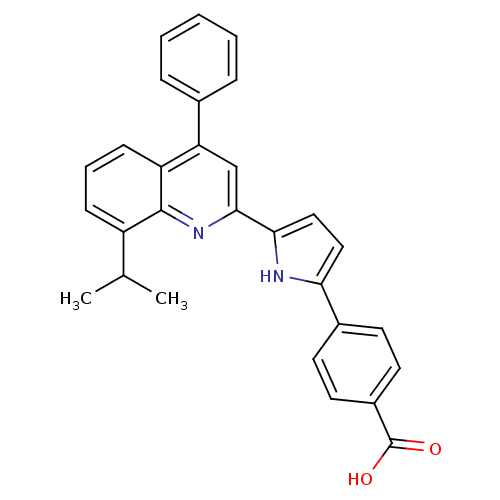
(4-[5-(8-Isopropyl-4-phenyl-quinolin-2-yl)-1H-pyrro...)Show SMILES CC(C)c1cccc2c(cc(nc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C29H24N2O2/c1-18(2)22-9-6-10-23-24(19-7-4-3-5-8-19)17-27(31-28(22)23)26-16-15-25(30-26)20-11-13-21(14-12-20)29(32)33/h3-18,30H,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor alpha transactivation by ATRA (50 nM) |
Bioorg Med Chem Lett 11: 1215-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M3643 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50029774
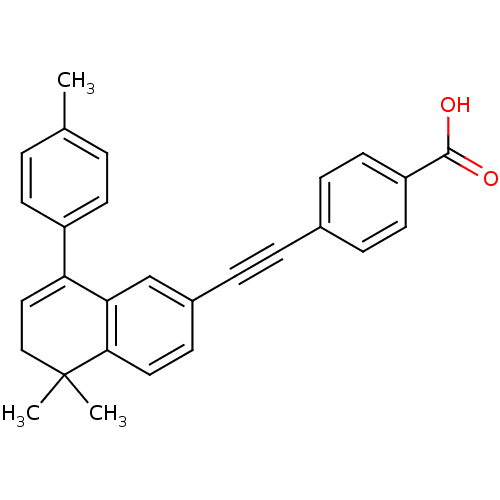
(4-(5,5-Dimethyl-8-p-tolyl-5,6-dihydro-naphthalen-2...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O |t:8| Show InChI InChI=1S/C28H24O2/c1-19-4-11-22(12-5-19)24-16-17-28(2,3)26-15-10-21(18-25(24)26)7-6-20-8-13-23(14-9-20)27(29)30/h4-5,8-16,18H,17H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor alpha |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084831

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(o1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219
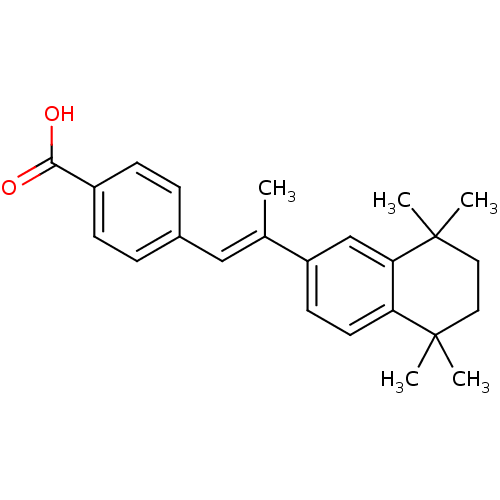
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084836
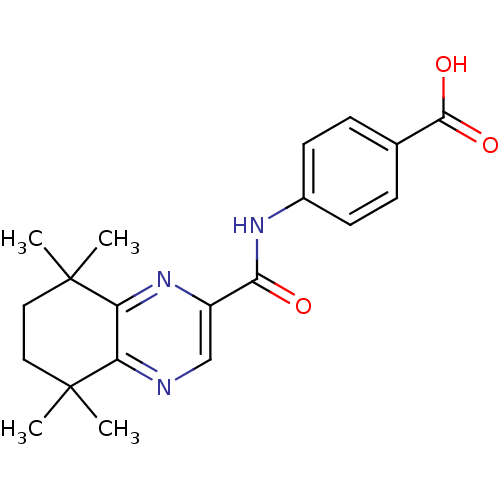
(4-[(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quinoxa...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H23N3O3/c1-19(2)9-10-20(3,4)16-15(19)21-11-14(23-16)17(24)22-13-7-5-12(6-8-13)18(25)26/h5-8,11H,9-10H2,1-4H3,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha). |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052414

(4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-15(7-10-17(18)21)19(24)23-16-8-5-14(6-9-16)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625
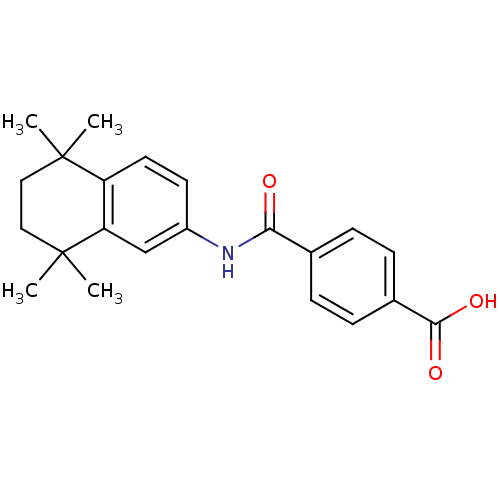
(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084830
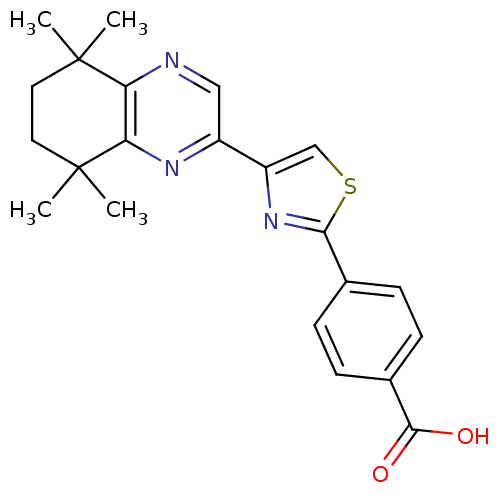
(4-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1csc(n1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H23N3O2S/c1-21(2)9-10-22(3,4)18-17(21)23-11-15(24-18)16-12-28-19(25-16)13-5-7-14(8-6-13)20(26)27/h5-8,11-12H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50044099
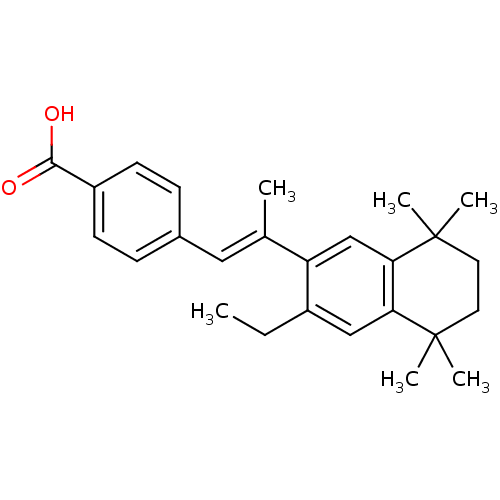
(4-[(E)-2-(3-Ethyl-5,5,8,8-tetramethyl-5,6,7,8-tetr...)Show SMILES CCc1cc2c(cc1\C(C)=C\c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O2/c1-7-19-15-22-23(26(5,6)13-12-25(22,3)4)16-21(19)17(2)14-18-8-10-20(11-9-18)24(27)28/h8-11,14-16H,7,12-13H2,1-6H3,(H,27,28)/b17-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50447840

(BMS-204493 | CHEMBL472172)Show SMILES CC1(C)CC=C(C#Cc2ccccc2)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 |t:4| Show InChI InChI=1S/C29H24O2/c1-29(2)19-18-24(14-10-21-6-4-3-5-7-21)26-20-23(13-17-27(26)29)9-8-22-11-15-25(16-12-22)28(30)31/h3-9,11-13,15-18,20H,19H2,1-2H3,(H,30,31)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RARalpha (unknown origin) |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084829
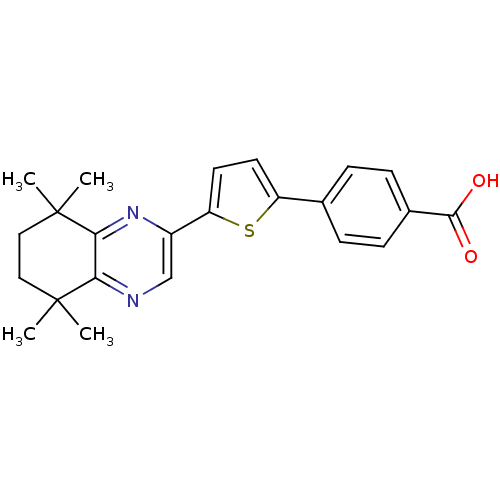
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(s1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50097823
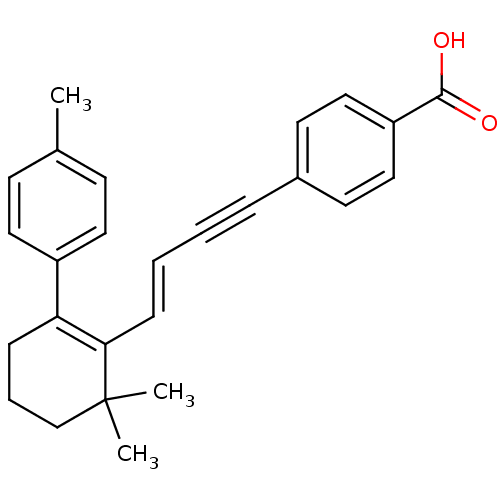
(4-[(E)-4-(6,6-Dimethyl-2-p-tolyl-cyclohex-1-enyl)-...)Show SMILES Cc1ccc(cc1)C1=C(\C=C\C#Cc2ccc(cc2)C(O)=O)C(C)(C)CCC1 |c:8| Show InChI InChI=1S/C26H26O2/c1-19-10-14-21(15-11-19)23-8-6-18-26(2,3)24(23)9-5-4-7-20-12-16-22(17-13-20)25(27)28/h5,9-17H,6,8,18H2,1-3H3,(H,27,28)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor gamma |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50097821
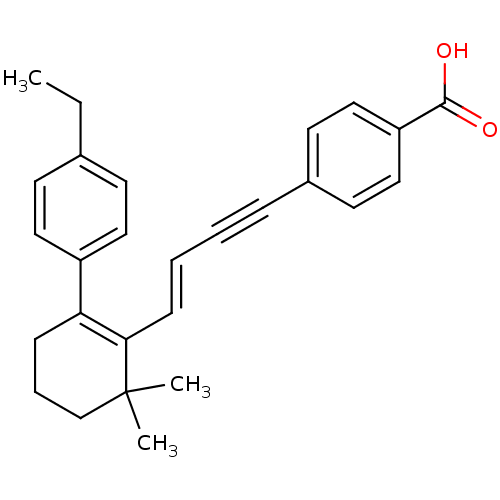
(4-{(E)-4-[2-(4-Ethyl-phenyl)-6,6-dimethyl-cyclohex...)Show SMILES CCc1ccc(cc1)C1=C(\C=C\C#Cc2ccc(cc2)C(O)=O)C(C)(C)CCC1 |c:9| Show InChI InChI=1S/C27H28O2/c1-4-20-11-15-22(16-12-20)24-9-7-19-27(2,3)25(24)10-6-5-8-21-13-17-23(18-14-21)26(28)29/h6,10-18H,4,7,9,19H2,1-3H3,(H,28,29)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor alpha |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50097820
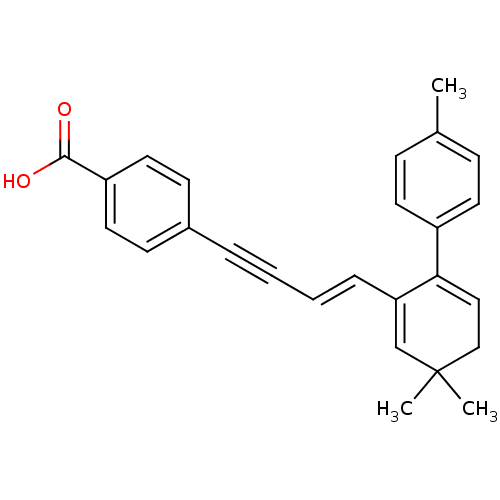
(4-[(E)-4-(3,3-Dimethyl-6-p-tolyl-cyclohexa-1,5-die...)Show SMILES Cc1ccc(cc1)C1=CCC(C)(C)C=C1\C=C\C#Cc1ccc(cc1)C(O)=O |c:14,t:8| Show InChI InChI=1S/C26H24O2/c1-19-8-12-21(13-9-19)24-16-17-26(2,3)18-23(24)7-5-4-6-20-10-14-22(15-11-20)25(27)28/h5,7-16,18H,17H2,1-3H3,(H,27,28)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor alpha |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032221
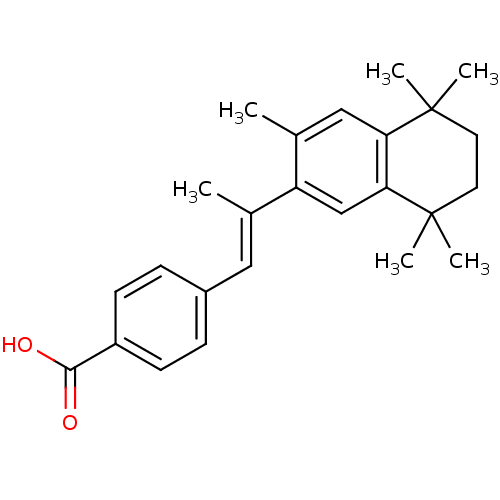
(4-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H30O2/c1-16(13-18-7-9-19(10-8-18)23(26)27)20-15-22-21(14-17(20)2)24(3,4)11-12-25(22,5)6/h7-10,13-15H,11-12H2,1-6H3,(H,26,27)/b16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 638 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50097822
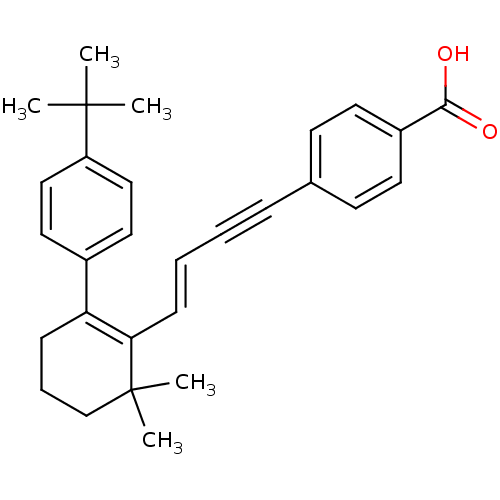
(4-{(E)-4-[2-(4-tert-Butyl-phenyl)-6,6-dimethyl-cyc...)Show SMILES CC(C)(C)c1ccc(cc1)C1=C(\C=C\C#Cc2ccc(cc2)C(O)=O)C(C)(C)CCC1 |c:11| Show InChI InChI=1S/C29H32O2/c1-28(2,3)24-18-16-22(17-19-24)25-10-8-20-29(4,5)26(25)11-7-6-9-21-12-14-23(15-13-21)27(30)31/h7,11-19H,8,10,20H2,1-5H3,(H,30,31)/b11-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 875 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Ability to inhibit TTNPB-induced transactivation at retinoic acid receptor alpha |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143821
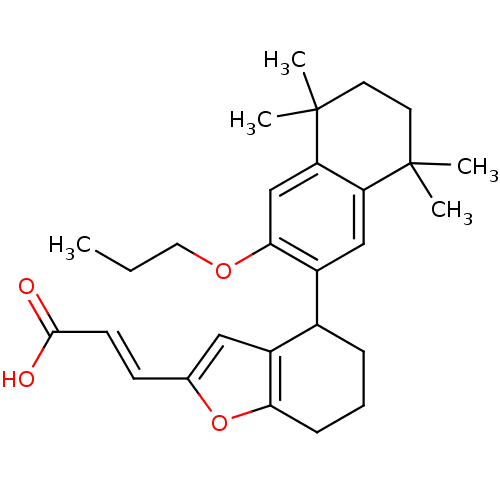
((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...)Show SMILES CCCOc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C28H36O4/c1-6-14-31-25-17-23-22(27(2,3)12-13-28(23,4)5)16-21(25)19-8-7-9-24-20(19)15-18(32-24)10-11-26(29)30/h10-11,15-17,19H,6-9,12-14H2,1-5H3,(H,29,30)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration for lipogenesis induced by retinoic acid receptor alpha in C3H10T1/2 clone 8 fibroblast cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50044098
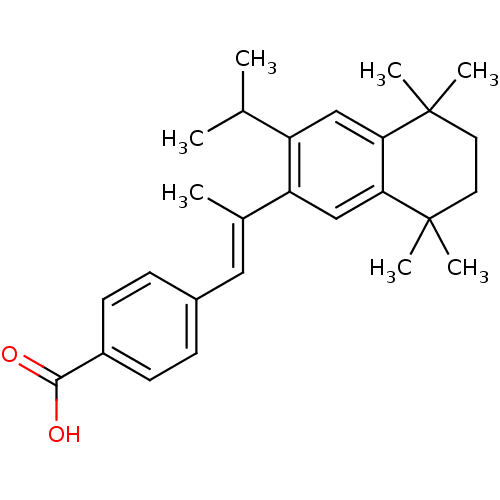
(4-[(E)-2-(3-Isopropyl-5,5,8,8-tetramethyl-5,6,7,8-...)Show SMILES CC(C)c1cc2c(cc1\C(C)=C\c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C27H34O2/c1-17(2)21-15-23-24(27(6,7)13-12-26(23,4)5)16-22(21)18(3)14-19-8-10-20(11-9-19)25(28)29/h8-11,14-17H,12-13H2,1-7H3,(H,28,29)/b18-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50447837
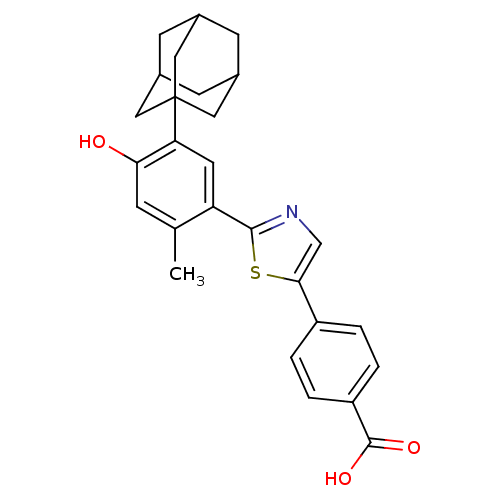
(CHEMBL3114297)Show SMILES Cc1cc(O)c(cc1-c1ncc(s1)-c1ccc(cc1)C(O)=O)C12CC3CC(CC(C3)C1)C2 |TLB:5:22:25:29.28.27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22| Show InChI InChI=1S/C27H27NO3S/c1-15-6-23(29)22(27-11-16-7-17(12-27)9-18(8-16)13-27)10-21(15)25-28-14-24(32-25)19-2-4-20(5-3-19)26(30)31/h2-6,10,14,16-18,29H,7-9,11-13H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi... |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50447838
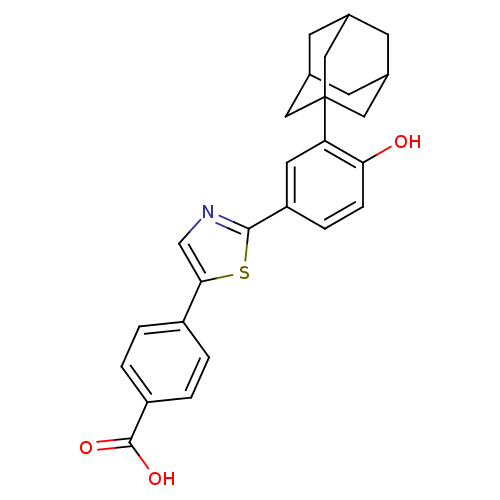
(CHEMBL3114296)Show SMILES OC(=O)c1ccc(cc1)-c1cnc(s1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |TLB:19:21:24:28.27.26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21| Show InChI InChI=1S/C26H25NO3S/c28-22-6-5-20(10-21(22)26-11-15-7-16(12-26)9-17(8-15)13-26)24-27-14-23(31-24)18-1-3-19(4-2-18)25(29)30/h1-6,10,14-17,28H,7-9,11-13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi... |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | CHEMBL5287875
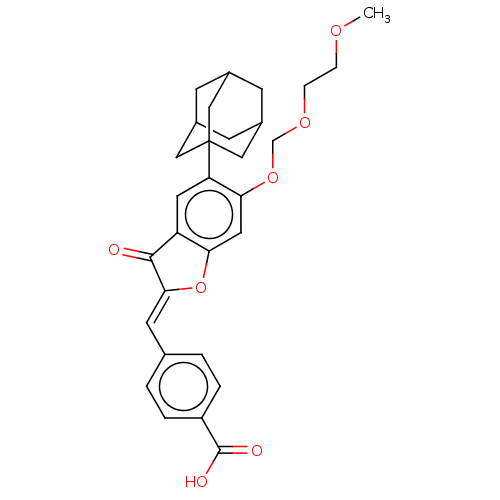
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50244760

((E)-4-{3-[3-Adamantan-1-yl-4-(2-methoxy-ethoxymeth...)Show SMILES COCCOCOc1ccc(cc1C12CC3CC(CC(C3)C1)C2)C(=O)\C=C\c1ccc(cc1)C(O)=O |TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13| Show InChI InChI=1S/C30H34O6/c1-34-10-11-35-19-36-28-9-7-25(27(31)8-4-20-2-5-24(6-3-20)29(32)33)15-26(28)30-16-21-12-22(17-30)14-23(13-21)18-30/h2-9,15,21-23H,10-14,16-19H2,1H3,(H,32,33)/b8-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi... |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50336730

(4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(cc1C(F)(F)F)C#N |w:5.4,c:9| Show InChI InChI=1S/C19H11F3N2O4S/c1-27-15-7-10(8-16-17(25)24-18(26)29-16)2-5-14(15)28-13-4-3-11(9-23)6-12(13)19(20,21)22/h2-8H,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at RARalpha by TR-FRET assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50158413
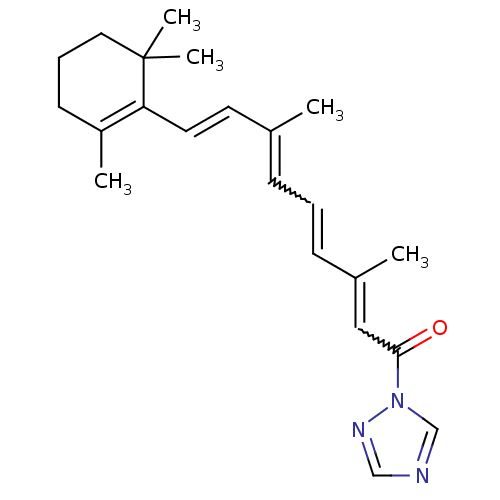
(CHEMBL388283 | N-(1H-1,2,4-triazol-1-yl)-(E)-retin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=CC(C)=CC(=O)n1cncn1 |w:14.14,18.19,c:4| Show InChI InChI=1S/C22H29N3O/c1-17(11-12-20-19(3)10-7-13-22(20,4)5)8-6-9-18(2)14-21(26)25-16-23-15-24-25/h6,8-9,11-12,14-16H,7,10,13H2,1-5H3/b9-6?,12-11+,17-8+,18-14? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50091698
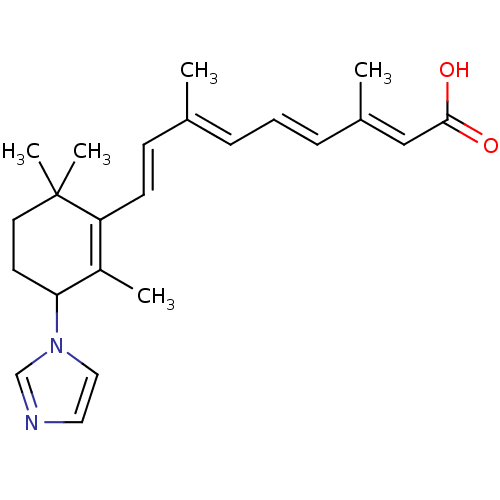
((2E,4E,6E,8E)-9-(3-Imidazol-1-yl-2,6,6-trimethyl-c...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C23H30N2O2/c1-17(7-6-8-18(2)15-22(26)27)9-10-20-19(3)21(11-12-23(20,4)5)25-14-13-24-16-25/h6-10,13-16,21H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,17-7+,18-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50158416
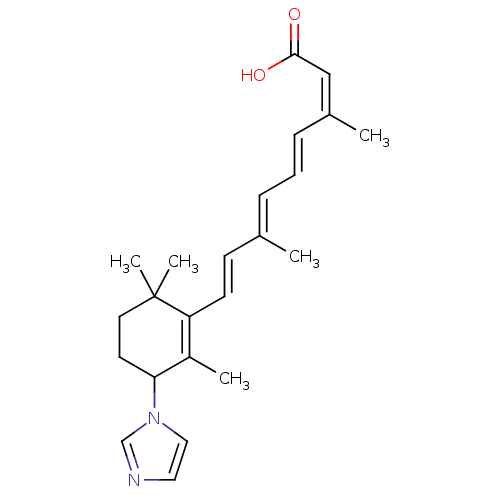
(4-((+/-)-(1H-imidazol-1-yl)-(13Z)-retinoic acid | ...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1ccnc1)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C23H30N2O2/c1-17(7-6-8-18(2)15-22(26)27)9-10-20-19(3)21(11-12-23(20,4)5)25-14-13-24-16-25/h6-10,13-16,21H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,17-7+,18-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50091699
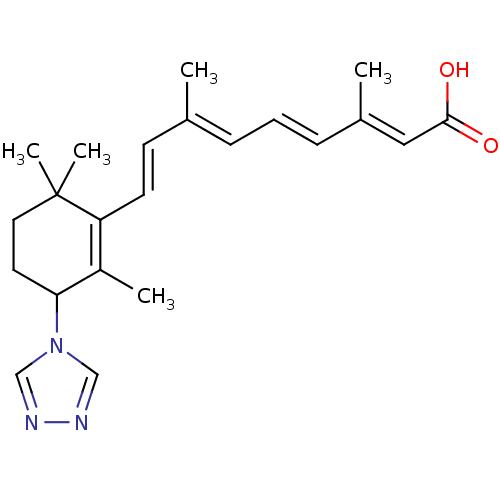
((2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-3-[1...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1cnnc1)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C22H29N3O2/c1-16(7-6-8-17(2)13-21(26)27)9-10-19-18(3)20(11-12-22(19,4)5)25-14-23-24-15-25/h6-10,13-15,20H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,16-7+,17-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50158412
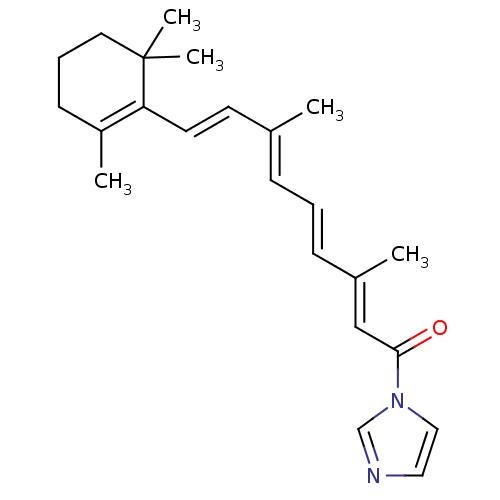
(CHEMBL388064 | N-(1H-imidazole-1-yl)-(E)-retinamid...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(=O)n1ccnc1 |c:4| Show InChI InChI=1S/C23H30N2O/c1-18(11-12-21-20(3)10-7-13-23(21,4)5)8-6-9-19(2)16-22(26)25-15-14-24-17-25/h6,8-9,11-12,14-17H,7,10,13H2,1-5H3/b9-6+,12-11+,18-8+,19-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50091700
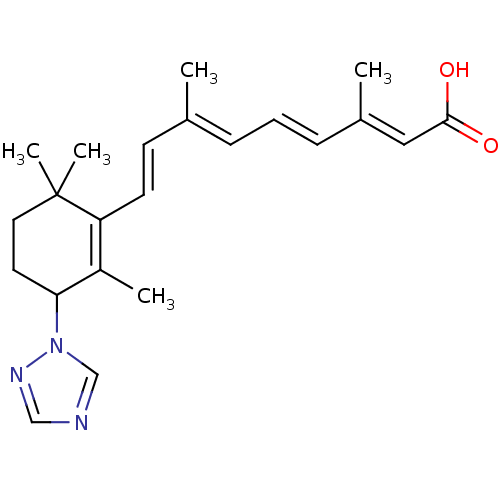
((2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-3-[1...)Show SMILES C\C(\C=C\C1=C(C)C(CCC1(C)C)n1cncn1)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C22H29N3O2/c1-16(7-6-8-17(2)13-21(26)27)9-10-19-18(3)20(11-12-22(19,4)5)25-15-23-14-24-25/h6-10,13-15,20H,11-12H2,1-5H3,(H,26,27)/b8-6+,10-9+,16-7+,17-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [11,12-3H]ARTA from RARalpha |
J Med Chem 47: 6716-29 (2004)
Article DOI: 10.1021/jm0401457
BindingDB Entry DOI: 10.7270/Q2K64HJV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data