 Found 36 hits of ec50 for UniProtKB: Q15139
Found 36 hits of ec50 for UniProtKB: Q15139 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519087
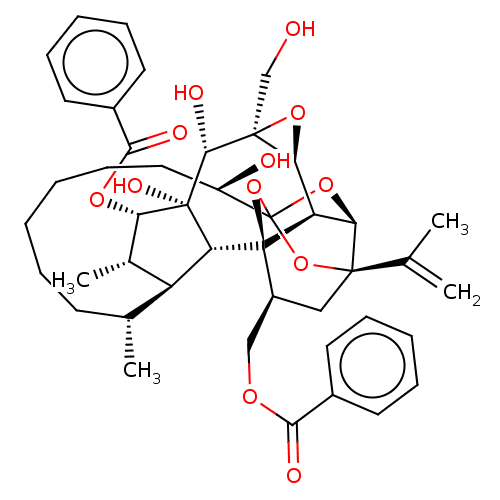
(CHEMBL3741746)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:35:54.55,THB:55:34:38:54.53.42,32:34:38:54.53.42,53:54:40.38.41:35,42:40:35:54.55,56:54:40.38.41:35| Show InChI InChI=1S/C44H54O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,39,45-46,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519063
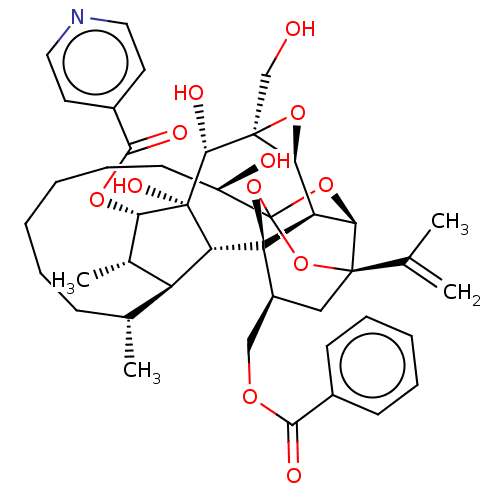
(CHEMBL4587471)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccncc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C43H53NO12/c1-23(2)39-20-28(21-51-36(47)26-13-9-7-10-14-26)42-31-34(39)54-43(55-39,56-42)29(46)15-11-6-5-8-12-24(3)30-25(4)33(52-37(48)27-16-18-44-19-17-27)41(50,32(30)42)38(49)40(22-45)35(31)53-40/h7,9-10,13-14,16-19,24-25,28-35,38,45-46,49-50H,1,5-6,8,11-12,15,20-22H2,2-4H3/t24-,25+,28+,29-,30+,31-,32-,33+,34-,35+,38-,39-,40+,41-,42-,43?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519068
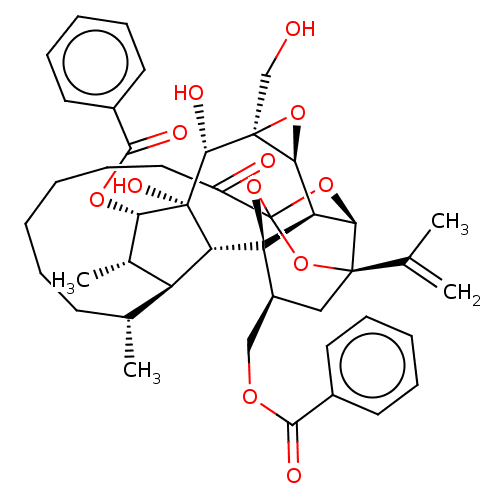
(CHEMBL4575056)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCCC(=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29,31-36,39,45,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519085

(CHEMBL4444766)Show SMILES [H][C@@]12O[C@]1(CO)C(=O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,45-46,50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.90 | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human U1 cells infected with HIV-1 NL4-3 assessed as induction of HIV-1 p24 production after 72 hrs by ELISA |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50099066
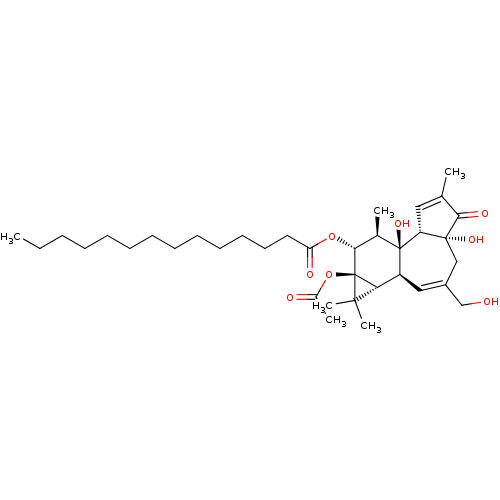
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324346

(CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C23H29N7/c1-2-4-20(5-3-1)28-22-13-17(6-7-25-22)21-12-18(19-15-26-27-16-19)14-23(29-21)30-10-8-24-9-11-30/h6-7,12-16,20,24H,1-5,8-11H2,(H,25,28)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324324

(2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2)c1)N1CCNCC1 Show InChI InChI=1S/C21H22N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h1-7,12-14,23H,8-11H2,(H2,22,28)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324331
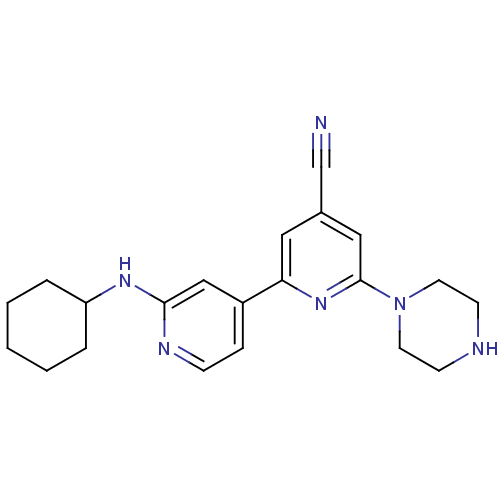
(2'-Cyclohexylamino-6-piperazin-1-yl[2,4']bipyridin...)Show SMILES N#Cc1cc(nc(c1)-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C21H26N6/c22-15-16-12-19(26-21(13-16)27-10-8-23-9-11-27)17-6-7-24-20(14-17)25-18-4-2-1-3-5-18/h6-7,12-14,18,23H,1-5,8-11H2,(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50470108
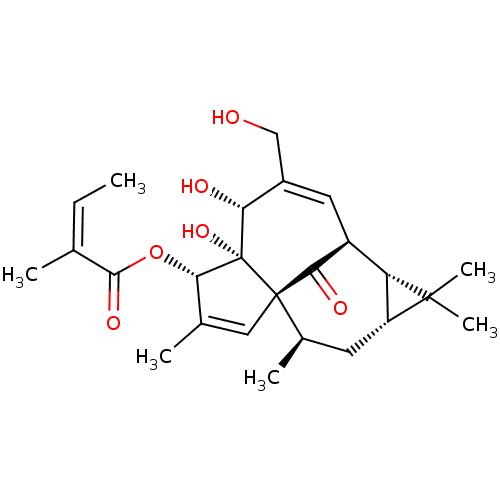
(AGN 204332 | Ingenol Mebutate | PEP-005 | PEP005 |...)Show SMILES [H][C@@]12C[C@@]([H])(C)[C@]34C=C(C)[C@]([H])(OC(=O)C(\C)=C/C)[C@@]3(O)[C@]([H])(O)C(CO)=C[C@]([H])(C4=O)[C@]1([H])C2(C)C |r,c:27,t:7| Show InChI InChI=1S/C25H34O6/c1-7-12(2)22(29)31-21-13(3)10-24-14(4)8-17-18(23(17,5)6)16(20(24)28)9-15(11-26)19(27)25(21,24)30/h7,9-10,14,16-19,21,26-27,30H,8,11H2,1-6H3/b12-7-/t14-,16+,17-,18+,19-,21+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324323
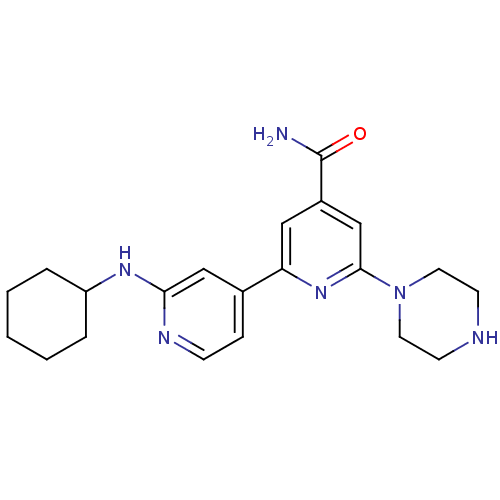
(2'-Cyclohexylamino-6-piperazin-1-yl[2,4']bipyridin...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C21H28N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h6-7,12-14,17,23H,1-5,8-11H2,(H2,22,28)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324347
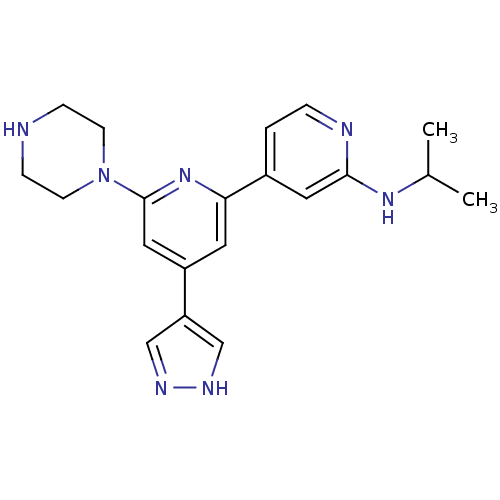
(CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C20H25N7/c1-14(2)25-19-10-15(3-4-22-19)18-9-16(17-12-23-24-13-17)11-20(26-18)27-7-5-21-6-8-27/h3-4,9-14,21H,5-8H2,1-2H3,(H,22,25)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324322
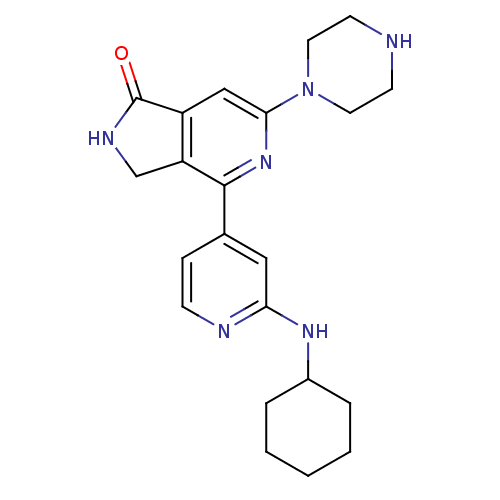
(4-(2-cyclohexylaminopyridin-4-yl)-6-(piperazin-1-y...)Show SMILES O=C1NCc2c1cc(nc2-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C22H28N6O/c29-22-17-13-20(28-10-8-23-9-11-28)27-21(18(17)14-25-22)15-6-7-24-19(12-15)26-16-4-2-1-3-5-16/h6-7,12-13,16,23H,1-5,8-11,14H2,(H,24,26)(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324329

(2'-Cyclopentylamino-6-piperazin-1-yl[2,4']bipyridi...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C20H26N6O/c21-20(27)15-11-17(25-19(13-15)26-9-7-22-8-10-26)14-5-6-23-18(12-14)24-16-3-1-2-4-16/h5-6,11-13,16,22H,1-4,7-10H2,(H2,21,27)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 202 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324348
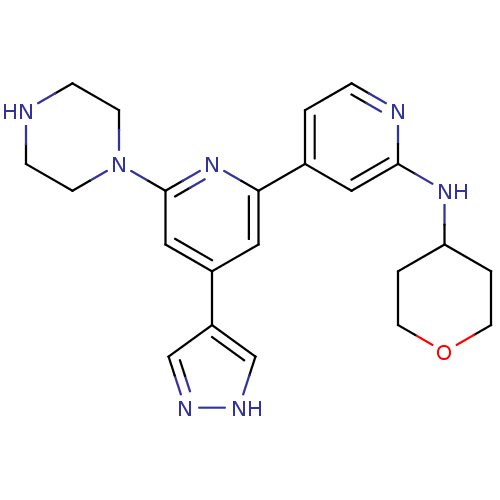
(6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...)Show SMILES C1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCOCC2)c1)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N7O/c1-4-24-21(27-19-2-9-30-10-3-19)12-16(1)20-11-17(18-14-25-26-15-18)13-22(28-20)29-7-5-23-6-8-29/h1,4,11-15,19,23H,2-3,5-10H2,(H,24,27)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 234 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324344
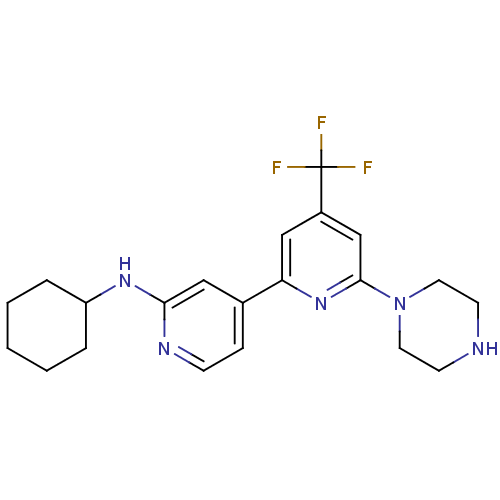
(CHEMBL1215360 | Cyclohexyl-(6-piperazin-1-yl-4-tri...)Show SMILES FC(F)(F)c1cc(nc(c1)-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C21H26F3N5/c22-21(23,24)16-13-18(28-20(14-16)29-10-8-25-9-11-29)15-6-7-26-19(12-15)27-17-4-2-1-3-5-17/h6-7,12-14,17,25H,1-5,8-11H2,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 342 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324332
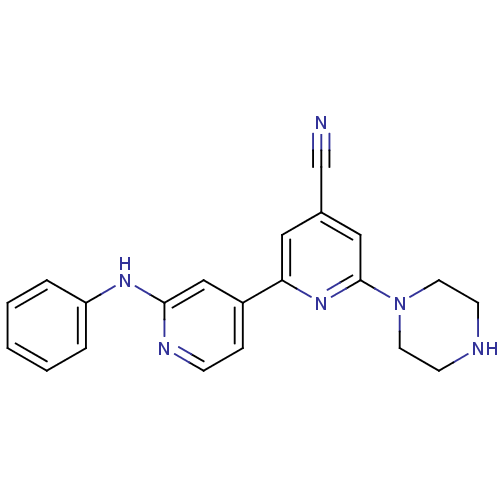
(2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...)Show SMILES N#Cc1cc(nc(c1)-c1ccnc(Nc2ccccc2)c1)N1CCNCC1 Show InChI InChI=1S/C21H20N6/c22-15-16-12-19(26-21(13-16)27-10-8-23-9-11-27)17-6-7-24-20(14-17)25-18-4-2-1-3-5-18/h1-7,12-14,23H,8-11H2,(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 378 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324333
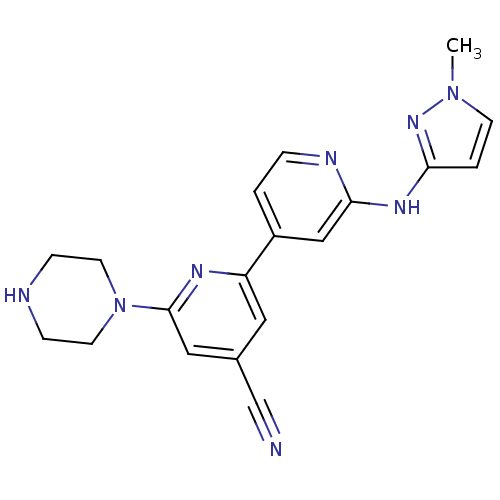
(2'-(1-Methyl-1H-pyrazol-3-ylamino)-6-piperazin-1-y...)Show SMILES Cn1ccc(Nc2cc(ccn2)-c2cc(cc(n2)N2CCNCC2)C#N)n1 Show InChI InChI=1S/C19H20N8/c1-26-7-3-17(25-26)24-18-12-15(2-4-22-18)16-10-14(13-20)11-19(23-16)27-8-5-21-6-9-27/h2-4,7,10-12,21H,5-6,8-9H2,1H3,(H,22,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 428 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324326
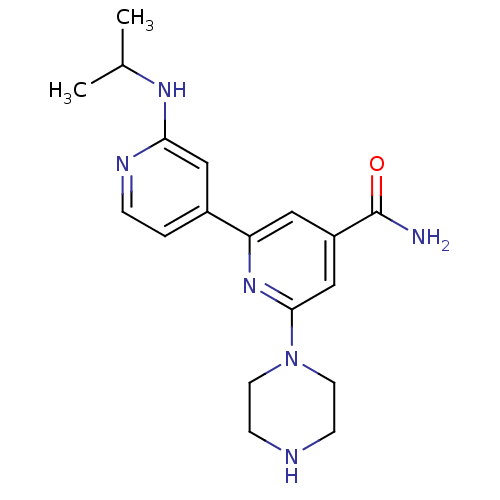
(4'-tert-Butylcarbamoyl-2''-isopropylamino-3,4,5,6-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)C(N)=O Show InChI InChI=1S/C18H24N6O/c1-12(2)22-16-10-13(3-4-21-16)15-9-14(18(19)25)11-17(23-15)24-7-5-20-6-8-24/h3-4,9-12,20H,5-8H2,1-2H3,(H2,19,25)(H,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 438 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324328
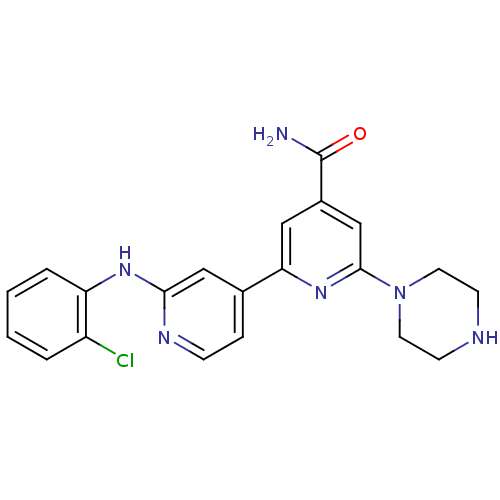
(2'-(2-Chlorophenylamino)-6-piperazin-1-yl[2,4']bip...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2Cl)c1)N1CCNCC1 Show InChI InChI=1S/C21H21ClN6O/c22-16-3-1-2-4-17(16)26-19-12-14(5-6-25-19)18-11-15(21(23)29)13-20(27-18)28-9-7-24-8-10-28/h1-6,11-13,24H,7-10H2,(H2,23,29)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 478 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324345

(CHEMBL1215361 | Cyclohexyl-(4-difluoromethyl-6-pip...)Show SMILES FC(F)c1cc(nc(c1)-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C21H27F2N5/c22-21(23)16-12-18(27-20(14-16)28-10-8-24-9-11-28)15-6-7-25-19(13-15)26-17-4-2-1-3-5-17/h6-7,12-14,17,21,24H,1-5,8-11H2,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 568 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324327
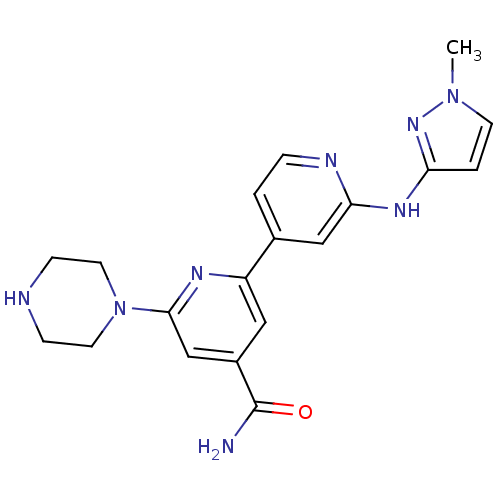
(2'-(1-Methyl-1H-pyrazol-3-ylamino)-6-piperazin-1-y...)Show SMILES Cn1ccc(Nc2cc(ccn2)-c2cc(cc(n2)N2CCNCC2)C(N)=O)n1 Show InChI InChI=1S/C19H22N8O/c1-26-7-3-16(25-26)24-17-11-13(2-4-22-17)15-10-14(19(20)28)12-18(23-15)27-8-5-21-6-9-27/h2-4,7,10-12,21H,5-6,8-9H2,1H3,(H2,20,28)(H,22,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 674 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324337
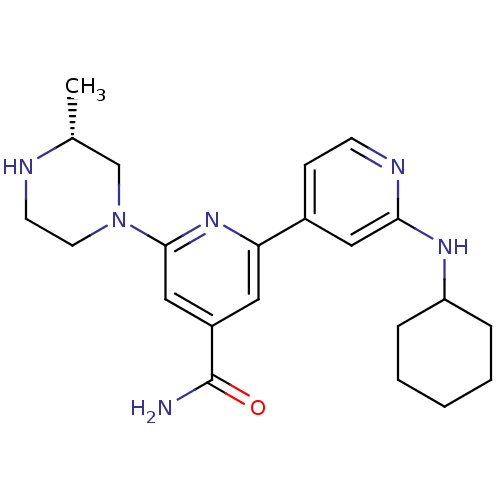
(2'-Cyclohexylamino-6-((R)-3-methylpiperazin-1-yl)[...)Show SMILES C[C@@H]1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCCCC2)c1)C(N)=O |r| Show InChI InChI=1S/C22H30N6O/c1-15-14-28(10-9-24-15)21-13-17(22(23)29)11-19(27-21)16-7-8-25-20(12-16)26-18-5-3-2-4-6-18/h7-8,11-13,15,18,24H,2-6,9-10,14H2,1H3,(H2,23,29)(H,25,26)/t15-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 679 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324334
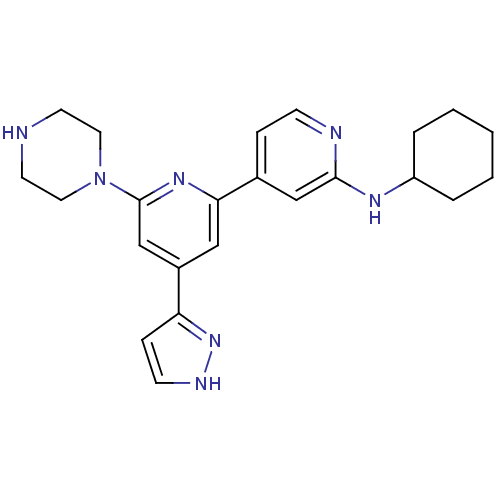
(CHEMBL1215152 | Cyclohexyl-[6-piperazin-1-yl-4-(2H...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cc[nH]n1 Show InChI InChI=1S/C23H29N7/c1-2-4-19(5-3-1)27-22-15-17(6-8-25-22)21-14-18(20-7-9-26-29-20)16-23(28-21)30-12-10-24-11-13-30/h6-9,14-16,19,24H,1-5,10-13H2,(H,25,27)(H,26,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 704 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324340
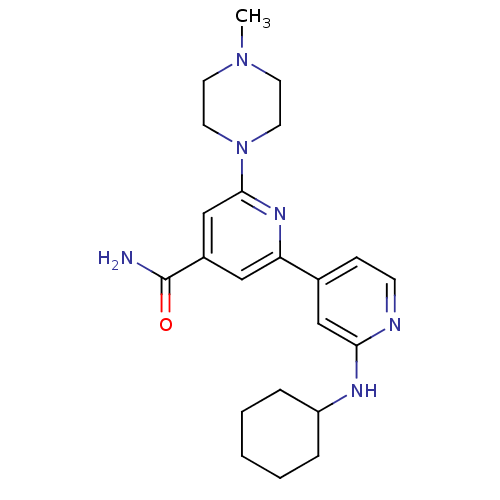
(2'-Cyclohexylamino-6-(4-methylpiperazin-1-yl)[2,4'...)Show SMILES CN1CCN(CC1)c1cc(cc(n1)-c1ccnc(NC2CCCCC2)c1)C(N)=O Show InChI InChI=1S/C22H30N6O/c1-27-9-11-28(12-10-27)21-15-17(22(23)29)13-19(26-21)16-7-8-24-20(14-16)25-18-5-3-2-4-6-18/h7-8,13-15,18H,2-6,9-12H2,1H3,(H2,23,29)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 752 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324338

(2'-Cyclohexylamino-6-((S)-3-methylpiperazin-1-yl)[...)Show SMILES C[C@H]1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCCCC2)c1)C(N)=O |r| Show InChI InChI=1S/C22H30N6O/c1-15-14-28(10-9-24-15)21-13-17(22(23)29)11-19(27-21)16-7-8-25-20(12-16)26-18-5-3-2-4-6-18/h7-8,11-13,15,18,24H,2-6,9-10,14H2,1H3,(H2,23,29)(H,25,26)/t15-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 831 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50368315

(PROSTRATIN)Show SMILES C[C@@H]1C[C@]2(OC(C)=O)[C@H]([C@@H]3C=C(CO)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]13O)C2(C)C |r,t:10,18| Show InChI InChI=1S/C22H30O6/c1-11-6-16-20(26,18(11)25)9-14(10-23)7-15-17-19(4,5)21(17,28-13(3)24)8-12(2)22(15,16)27/h6-7,12,15-17,23,26-27H,8-10H2,1-5H3/t12-,15+,16-,17?,20-,21+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324335
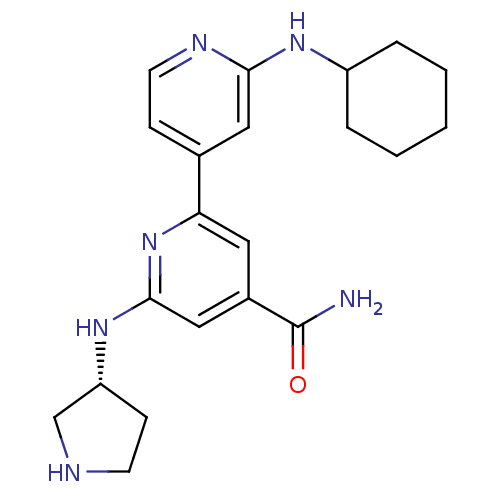
(2'-Cyclohexylamino-6-((R)-pyrrolidin-3-ylamino)[2,...)Show SMILES NC(=O)c1cc(N[C@@H]2CCNC2)nc(c1)-c1ccnc(NC2CCCCC2)c1 |r| Show InChI InChI=1S/C21H28N6O/c22-21(28)15-10-18(27-20(12-15)26-17-7-8-23-13-17)14-6-9-24-19(11-14)25-16-4-2-1-3-5-16/h6,9-12,16-17,23H,1-5,7-8,13H2,(H2,22,28)(H,24,25)(H,26,27)/t17-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324339
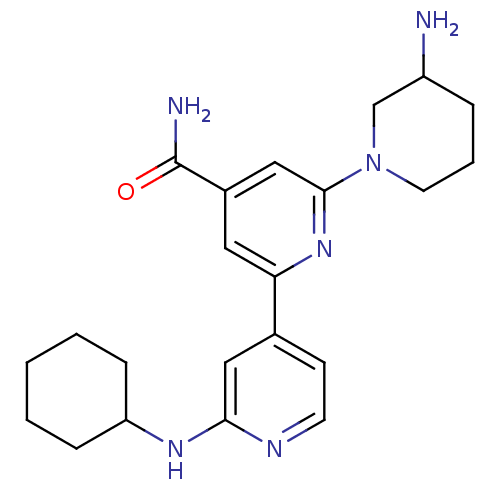
(3-Amino-2''-cyclohexylamino-3,4,5,6-tetrahydro-2H-...)Show SMILES NC1CCCN(C1)c1cc(cc(n1)-c1ccnc(NC2CCCCC2)c1)C(N)=O Show InChI InChI=1S/C22H30N6O/c23-17-5-4-10-28(14-17)21-13-16(22(24)29)11-19(27-21)15-8-9-25-20(12-15)26-18-6-2-1-3-7-18/h8-9,11-13,17-18H,1-7,10,14,23H2,(H2,24,29)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324336
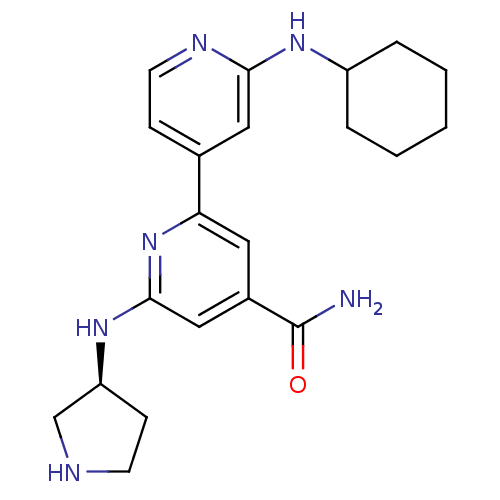
(2'-Cyclohexylamino-6-((S)-pyrrolidin-3-ylamino)[2,...)Show SMILES NC(=O)c1cc(N[C@H]2CCNC2)nc(c1)-c1ccnc(NC2CCCCC2)c1 |r| Show InChI InChI=1S/C21H28N6O/c22-21(28)15-10-18(27-20(12-15)26-17-7-8-23-13-17)14-6-9-24-19(11-14)25-16-4-2-1-3-5-16/h6,9-12,16-17,23H,1-5,7-8,13H2,(H2,22,28)(H,24,25)(H,26,27)/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324341
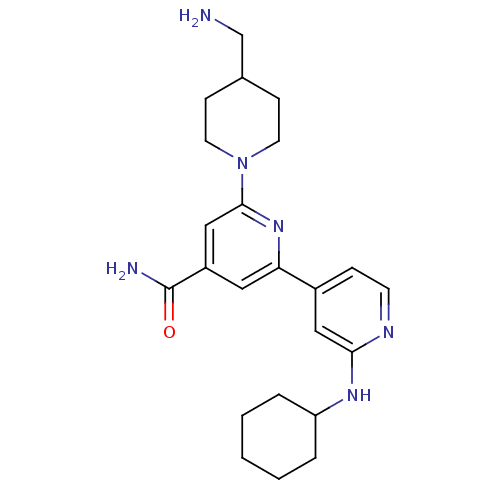
(Aminomethyl-2''-cyclohexylamino-3,4,5,6-tetrahydro...)Show SMILES NCC1CCN(CC1)c1cc(cc(n1)-c1ccnc(NC2CCCCC2)c1)C(N)=O Show InChI InChI=1S/C23H32N6O/c24-15-16-7-10-29(11-8-16)22-14-18(23(25)30)12-20(28-22)17-6-9-26-21(13-17)27-19-4-2-1-3-5-19/h6,9,12-14,16,19H,1-5,7-8,10-11,15,24H2,(H2,25,30)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324321
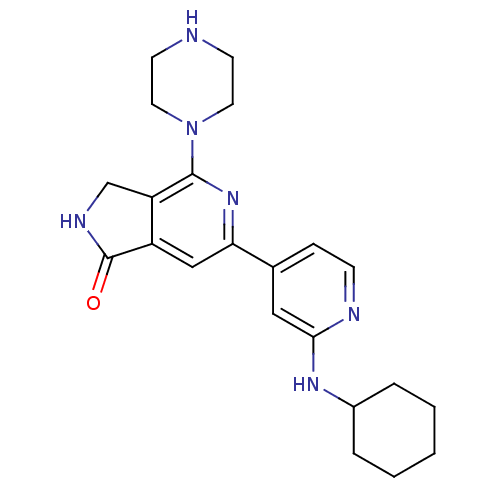
(6-(2-Cyclohexylaminopyridin-4-yl)-4-piperazin-1-yl...)Show SMILES O=C1NCc2c1cc(nc2N1CCNCC1)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C22H28N6O/c29-22-17-13-19(27-21(18(17)14-25-22)28-10-8-23-9-11-28)15-6-7-24-20(12-15)26-16-4-2-1-3-5-16/h6-7,12-13,16,23H,1-5,8-11,14H2,(H,24,26)(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324342
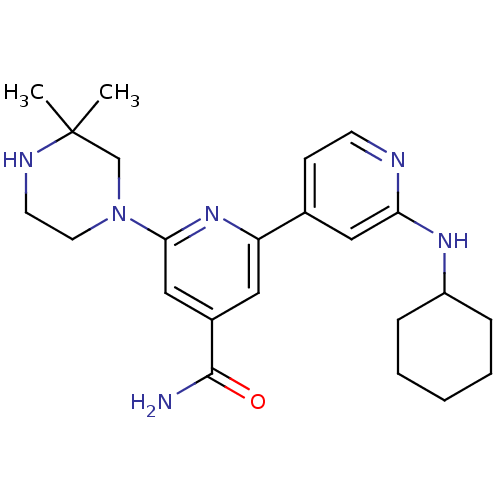
(2'-Cyclohexylamino-6-(3,3-dimethylpiperazin-1-yl)[...)Show SMILES CC1(C)CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCCCC2)c1)C(N)=O Show InChI InChI=1S/C23H32N6O/c1-23(2)15-29(11-10-26-23)21-14-17(22(24)30)12-19(28-21)16-8-9-25-20(13-16)27-18-6-4-3-5-7-18/h8-9,12-14,18,26H,3-7,10-11,15H2,1-2H3,(H2,24,30)(H,25,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324330
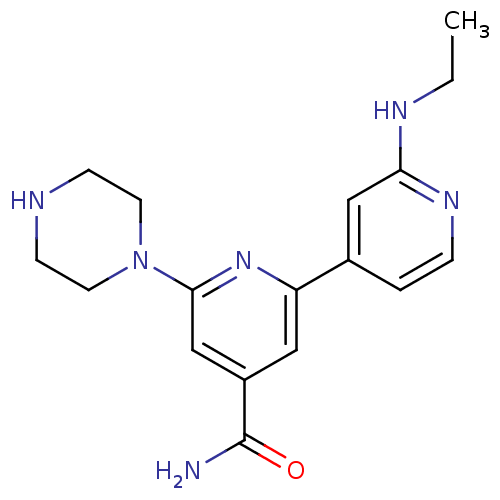
(2'-Ethylamino-6-piperazin-1-yl[2,4']bipyridinyl-4-...)Show InChI InChI=1S/C17H22N6O/c1-2-20-15-10-12(3-4-21-15)14-9-13(17(18)24)11-16(22-14)23-7-5-19-6-8-23/h3-4,9-11,19H,2,5-8H2,1H3,(H2,18,24)(H,20,21) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324325
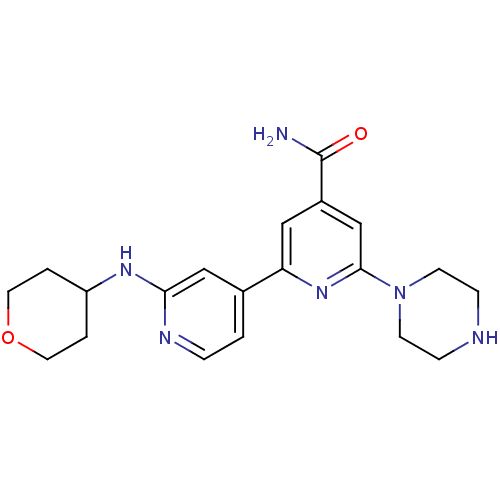
(6-Piperazin-1-yl-2'-(tetrahydropyran-4-ylamino)[2,...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCOCC2)c1)N1CCNCC1 Show InChI InChI=1S/C20H26N6O2/c21-20(27)15-11-17(25-19(13-15)26-7-5-22-6-8-26)14-1-4-23-18(12-14)24-16-2-9-28-10-3-16/h1,4,11-13,16,22H,2-3,5-10H2,(H2,21,27)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear export |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50470110

(CHEMBL4071723)Show SMILES [H][C@@]12C[C@@H](C)[C@]34C=C(C)[C@H](OC(=O)C(\C)=C/C)[C@@]3(O)[C@H](O)C(C)=C[C@]([H])(C4=O)[C@]1([H])C2(C)C |r,c:23,t:6| Show InChI InChI=1S/C25H34O5/c1-8-12(2)22(28)30-21-14(4)11-24-15(5)10-17-18(23(17,6)7)16(20(24)27)9-13(3)19(26)25(21,24)29/h8-9,11,15-19,21,26,29H,10H2,1-7H3/b12-8-/t15-,16+,17-,18+,19-,21+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50470109
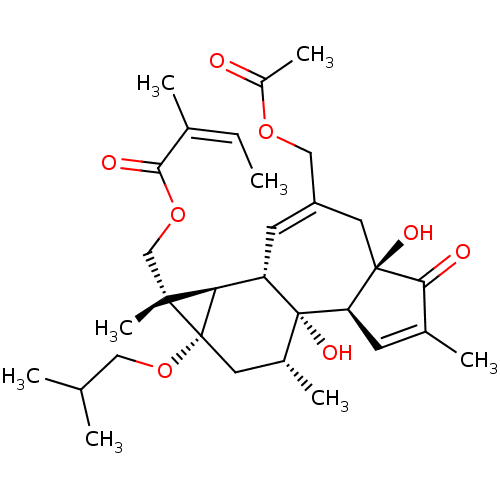
(CHEMBL4104319)Show SMILES [H][C@]12[C@]3([H])C=C(COC(C)=O)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)C[C@@]1(OCC(C)C)[C@]2(C)COC(=O)C(\C)=C/C |r,c:17,t:4| Show InChI InChI=1S/C31H44O8/c1-9-18(4)27(34)38-16-28(8)25-23-11-22(15-37-21(7)32)13-29(35)24(10-19(5)26(29)33)31(23,36)20(6)12-30(25,28)39-14-17(2)3/h9-11,17,20,23-25,35-36H,12-16H2,1-8H3/b18-9-/t20-,23+,24-,25-,28-,29-,30+,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.08E+3 | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Activation of PKC in human platelet assessed as induction of platelet aggregation measured within 15 mins by tribidimetric method |
J Nat Prod 79: 2658-2666 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00603
BindingDB Entry DOI: 10.7270/Q24T6N3M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data