 Found 32 hits of ki for UniProtKB: P06737
Found 32 hits of ki for UniProtKB: P06737 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148913
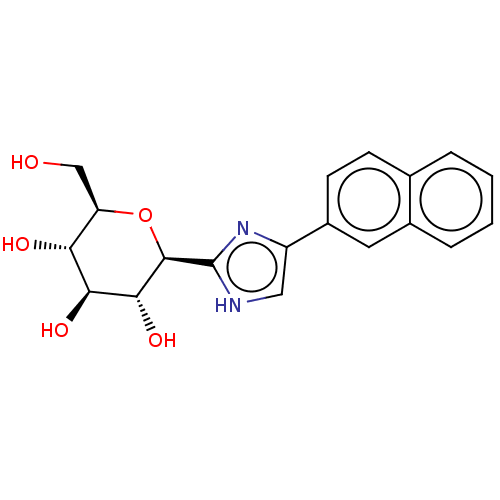
(CHEMBL3770455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)19-20-8-13(21-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50241632
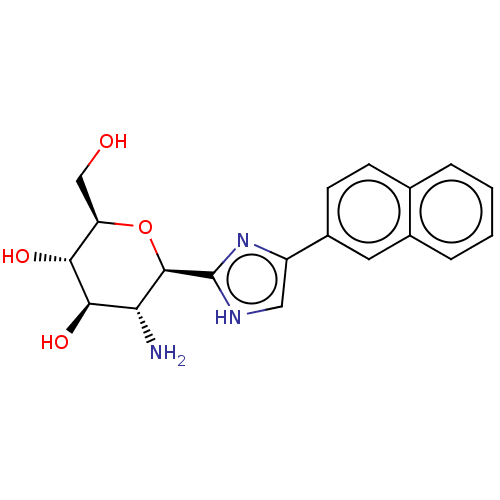
(CHEMBL4101331)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H21N3O4/c20-15-17(25)16(24)14(9-23)26-18(15)19-21-8-13(22-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,23-25H,9,20H2,(H,21,22)/t14-,15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inor... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148915
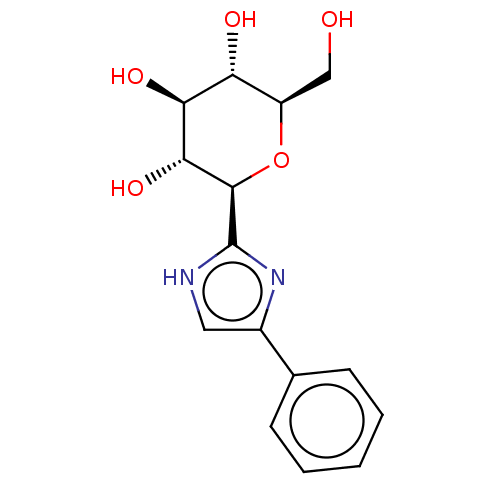
(CHEMBL3770514)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)15-16-6-9(17-15)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437354

(CHEMBL2408225)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437354

(CHEMBL2408225)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50016703
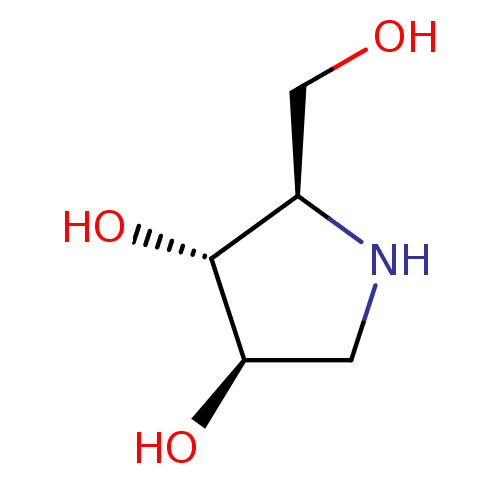
(2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory activity against pig Liver glycogen phosphorylase a |
J Med Chem 47: 3537-45 (2004)
Article DOI: 10.1021/jm031121n
BindingDB Entry DOI: 10.7270/Q2HM5977 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437355
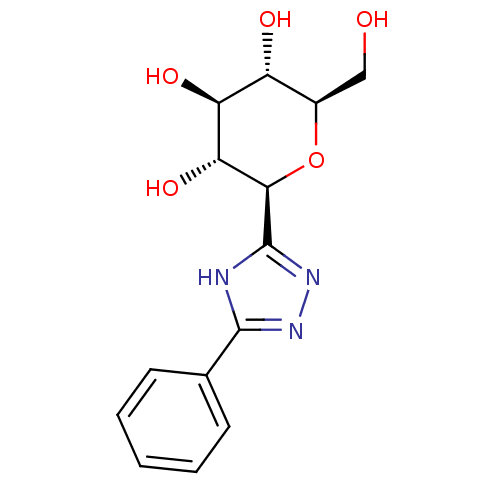
(CHEMBL2408224)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C14H17N3O5/c18-6-8-9(19)10(20)11(21)12(22-8)14-15-13(16-17-14)7-4-2-1-3-5-7/h1-5,8-12,18-21H,6H2,(H,15,16,17)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437355

(CHEMBL2408224)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C14H17N3O5/c18-6-8-9(19)10(20)11(21)12(22-8)14-15-13(16-17-14)7-4-2-1-3-5-7/h1-5,8-12,18-21H,6H2,(H,15,16,17)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of fibrinogen receptor |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312362
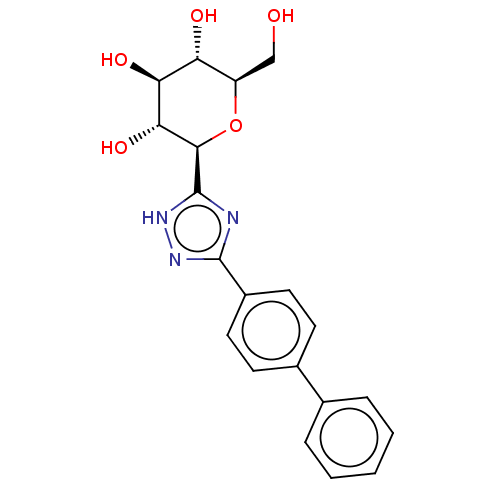
(CHEMBL4176460)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3O5/c24-10-14-15(25)16(26)17(27)18(28-14)20-21-19(22-23-20)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-18,24-27H,10H2,(H,21,22,23)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50313014

(CHEMBL4172670)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H21N3O7/c25-9-14-15(26)16(27)17(28)18(31-14)20-22-19(23-24-20)12-5-1-10(2-6-12)11-3-7-13(8-4-11)21(29)30/h1-8,14-18,25-28H,9H2,(H,29,30)(H,22,23,24)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50241633

(CHEMBL4095486)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O4/c16-11-13(21)12(20)10(7-19)22-14(11)15-17-6-9(18-15)8-4-2-1-3-5-8/h1-6,10-14,19-21H,7,16H2,(H,17,18)/t10-,11-,12-,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inor... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50538946
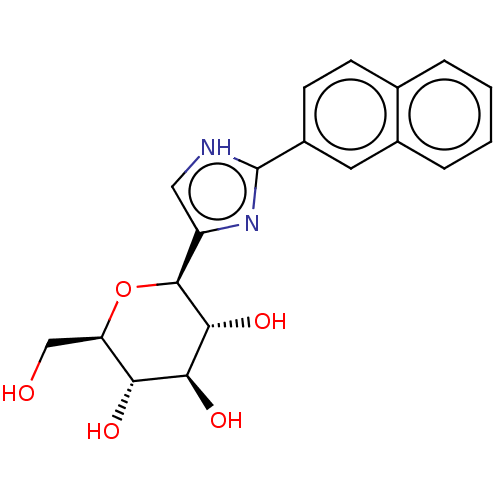
(CHEMBL4646575)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1c[nH]c(n1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)13-8-20-19(21-13)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312225
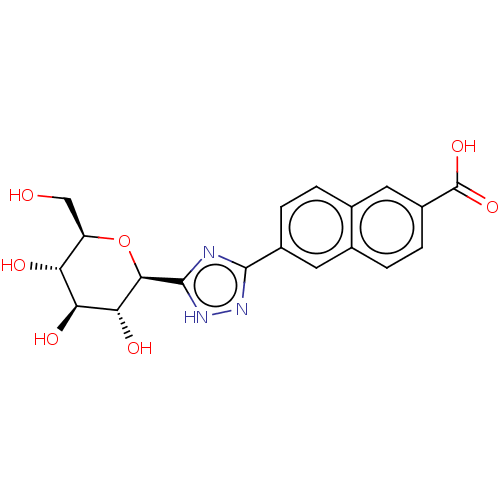
(CHEMBL4161353)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc2cc(ccc2c1)C(O)=O |r| Show InChI InChI=1S/C19H19N3O7/c23-7-12-13(24)14(25)15(26)16(29-12)18-20-17(21-22-18)10-3-1-9-6-11(19(27)28)4-2-8(9)5-10/h1-6,12-16,23-26H,7H2,(H,27,28)(H,20,21,22)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50211296
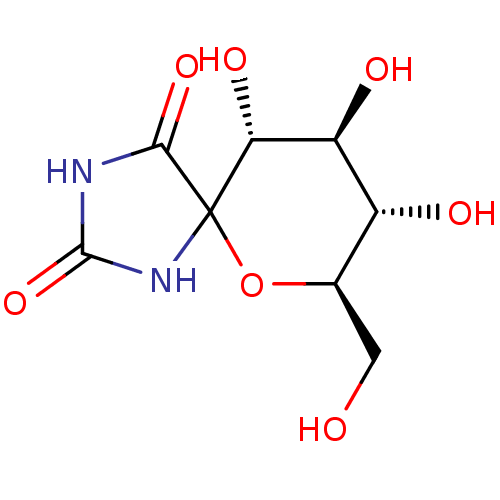
((7R,8S,9S,10R)-8,9,10-trihydroxy-7-hydroxymethyl-6...)Show SMILES OC[C@H]1OC2(NC(=O)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2-,3-,4+,5-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of liver glycogen phosphorylase B |
Bioorg Med Chem 15: 4048-56 (2007)
Article DOI: 10.1016/j.bmc.2007.03.084
BindingDB Entry DOI: 10.7270/Q2XW4JGC |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50211294
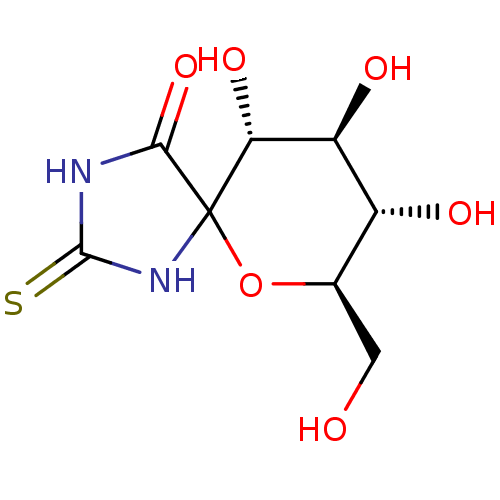
((7R,8S,9S,10R)-8,9,10-trihydroxy-7-hydroxymethyl-2...)Show SMILES OC[C@H]1OC2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of liver glycogen phosphorylase B |
Bioorg Med Chem 15: 4048-56 (2007)
Article DOI: 10.1016/j.bmc.2007.03.084
BindingDB Entry DOI: 10.7270/Q2XW4JGC |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50241634
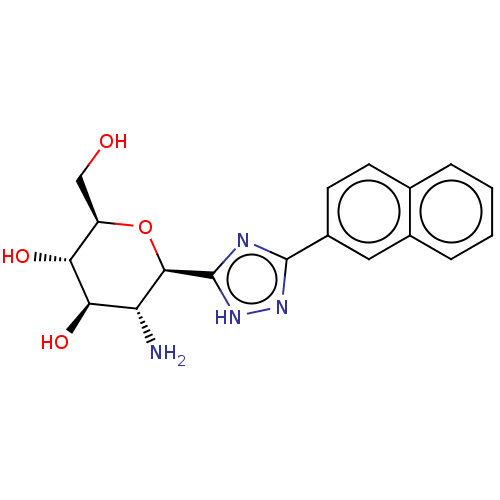
(CHEMBL4068103)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(n[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H20N4O4/c19-13-15(25)14(24)12(8-23)26-16(13)18-20-17(21-22-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,23-25H,8,19H2,(H,20,21,22)/t12-,13-,14-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inor... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312632

(CHEMBL4164542)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1cccc2ccccc12 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-7-3-5-9-4-1-2-6-10(9)11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50263769
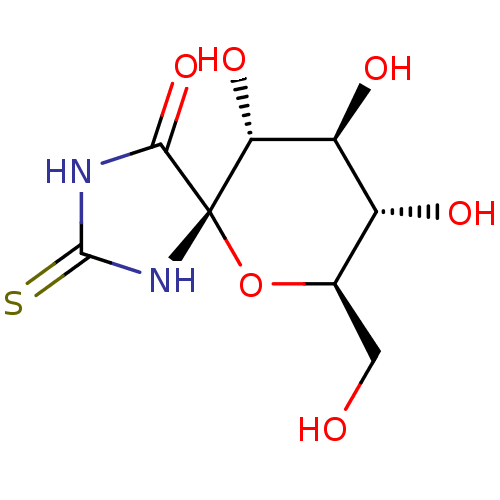
((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase a |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50211289
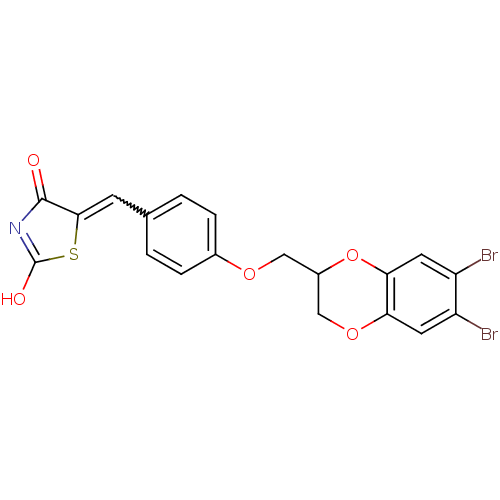
(5-[4-(6,7-dibromo-2,3-dihydrobenzo[1,4]dioxin-2-yl...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(OCC2COc3cc(Br)c(Br)cc3O2)cc1 |w:7.8,t:1| Show InChI InChI=1S/C19H13Br2NO5S/c20-13-6-15-16(7-14(13)21)27-12(9-26-15)8-25-11-3-1-10(2-4-11)5-17-18(23)22-19(24)28-17/h1-7,12H,8-9H2,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of liver glycogen phosphorylase B |
Bioorg Med Chem 15: 4048-56 (2007)
Article DOI: 10.1016/j.bmc.2007.03.084
BindingDB Entry DOI: 10.7270/Q2XW4JGC |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50538945

(CHEMBL4638064)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1c[nH]c(n1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)9-6-16-15(17-9)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50263769

((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against liver Glycogen Phosphorylase a |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50211288
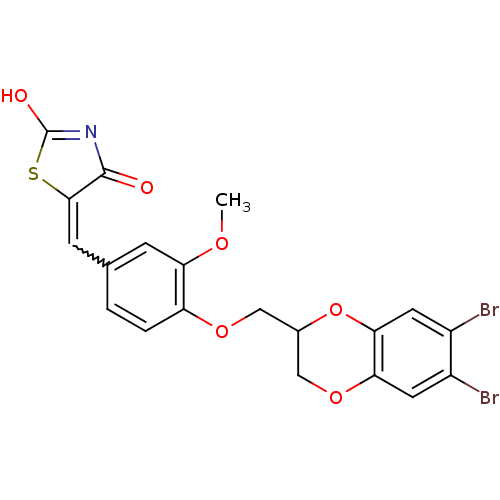
(5-[4-(6,7-dibromo-2,3-dihydrobenzo[1,4]dioxin-2-yl...)Show SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1OCC1COc2cc(Br)c(Br)cc2O1 |w:5.4,c:9| Show InChI InChI=1S/C20H15Br2NO6S/c1-26-15-4-10(5-18-19(24)23-20(25)30-18)2-3-14(15)27-8-11-9-28-16-6-12(21)13(22)7-17(16)29-11/h2-7,11H,8-9H2,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of liver glycogen phosphorylase B |
Bioorg Med Chem 15: 4048-56 (2007)
Article DOI: 10.1016/j.bmc.2007.03.084
BindingDB Entry DOI: 10.7270/Q2XW4JGC |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50241635
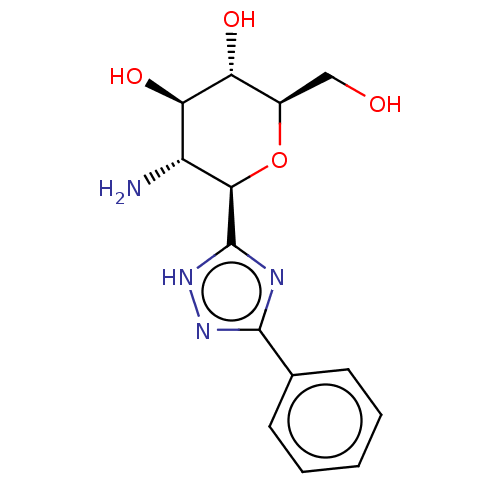
(CHEMBL4087784)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1c1nc(n[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C14H18N4O4/c15-9-11(21)10(20)8(6-19)22-12(9)14-16-13(17-18-14)7-4-2-1-3-5-7/h1-5,8-12,19-21H,6,15H2,(H,16,17,18)/t8-,9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inor... |
J Med Chem 60: 9251-9262 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01056
BindingDB Entry DOI: 10.7270/Q2ZC851N |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50538948
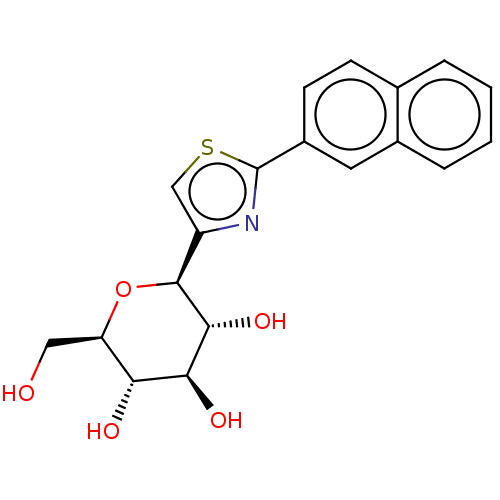
(CHEMBL4648992)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1csc(n1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H19NO5S/c21-8-14-15(22)16(23)17(24)18(25-14)13-9-26-19(20-13)12-6-5-10-3-1-2-4-11(10)7-12/h1-7,9,14-18,21-24H,8H2/t14-,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148917
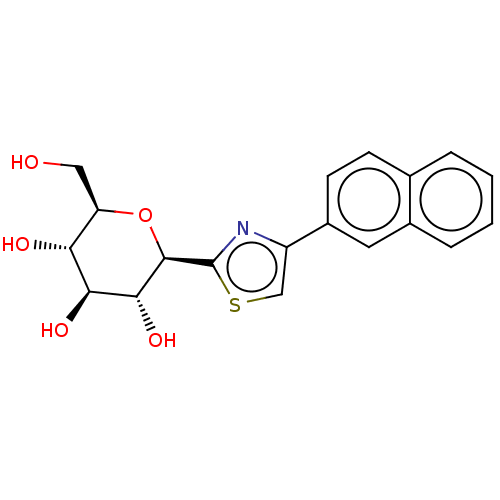
(CHEMBL3770888)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(cs1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H19NO5S/c21-8-14-15(22)16(23)17(24)18(25-14)19-20-13(9-26-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-7,9,14-18,21-24H,8H2/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50211286
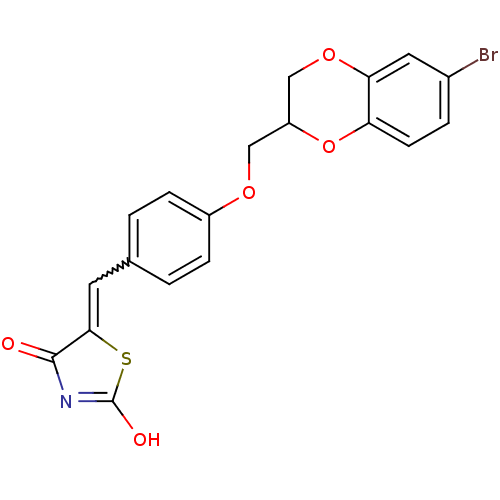
(5-[4-(6-bromo-2,3-dihydrobenzo[1,4]dioxin-2-ylmeth...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(OCC2COc3cc(Br)ccc3O2)cc1 |w:7.8,t:1| Show InChI InChI=1S/C19H14BrNO5S/c20-12-3-6-15-16(8-12)25-10-14(26-15)9-24-13-4-1-11(2-5-13)7-17-18(22)21-19(23)27-17/h1-8,14H,9-10H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of liver glycogen phosphorylase B |
Bioorg Med Chem 15: 4048-56 (2007)
Article DOI: 10.1016/j.bmc.2007.03.084
BindingDB Entry DOI: 10.7270/Q2XW4JGC |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50538949
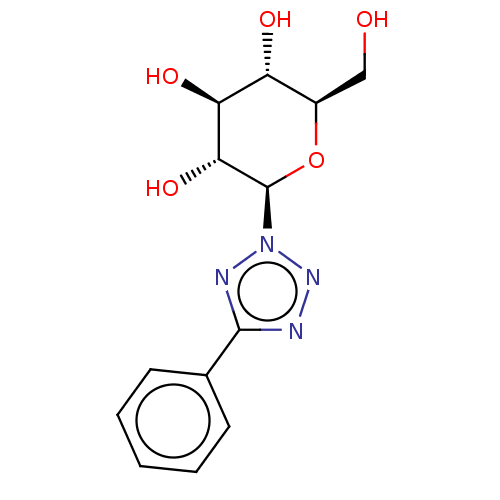
(CHEMBL4640451)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1nnc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C13H16N4O5/c18-6-8-9(19)10(20)11(21)13(22-8)17-15-12(14-16-17)7-4-2-1-3-5-7/h1-5,8-11,13,18-21H,6H2/t8-,9-,10+,11-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50538947
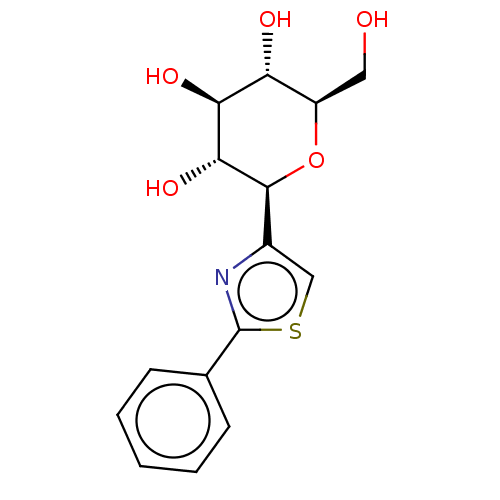
(CHEMBL4642474)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1csc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C15H17NO5S/c17-6-10-11(18)12(19)13(20)14(21-10)9-7-22-15(16-9)8-4-2-1-3-5-8/h1-5,7,10-14,17-20H,6H2/t10-,11-,12+,13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148914
(CHEMBL3769528)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C15H17NO5S/c17-6-10-11(18)12(19)13(20)14(21-10)15-16-9(7-22-15)8-4-2-1-3-5-8/h1-5,7,10-14,17-20H,6H2/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50351158
(CHEMBL423707)Show SMILES OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.28E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50351158

(CHEMBL423707)Show SMILES OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase a (unknown origin) |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data