

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50157689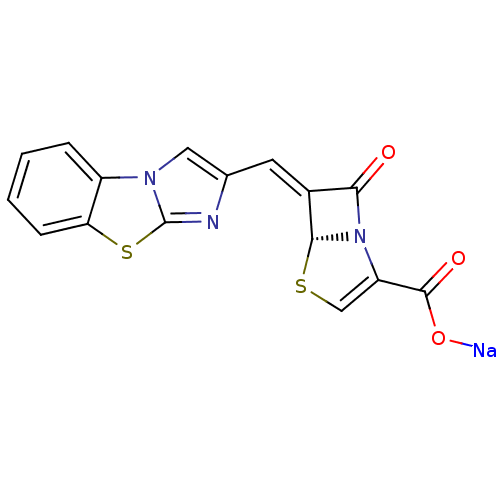 ((5R,6Z)-6-(imidazo[2,1-b][1,3]benzothiazol-2-ylmet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157688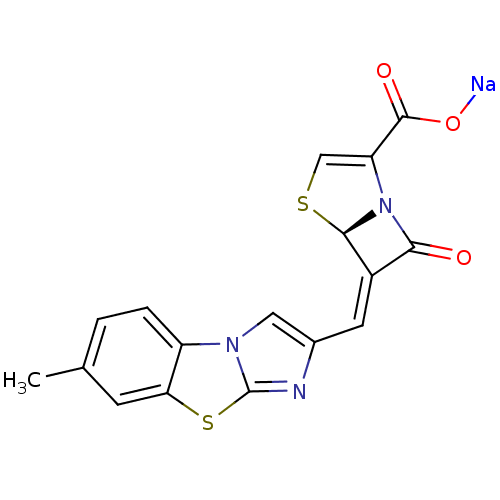 ((5R,6Z)-6-[(7-methylimidazo[2,1-b][1,3]-benzothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191379 ((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM467002 ((2R)-2-(((2S,5R)-2-cyano-4-methyl-7-oxo-1,6-diazab...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466971 (2-fluoro-2-(((2S,5R)-3-methyl-7-oxo-2-((pyrazin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466985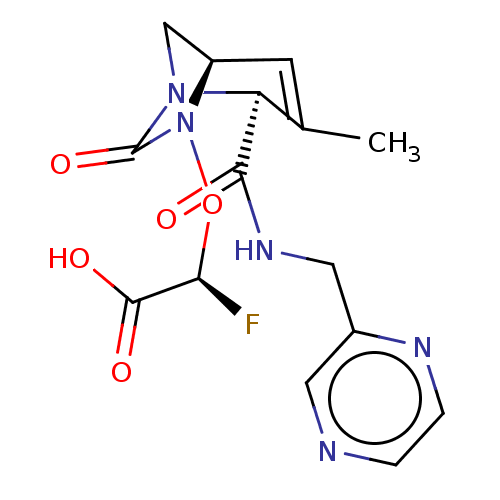 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(pyrazi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157694 ((5R,6Z)-6-[(7-fluoroimidazo[2,1-b][1,3]-benzothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157687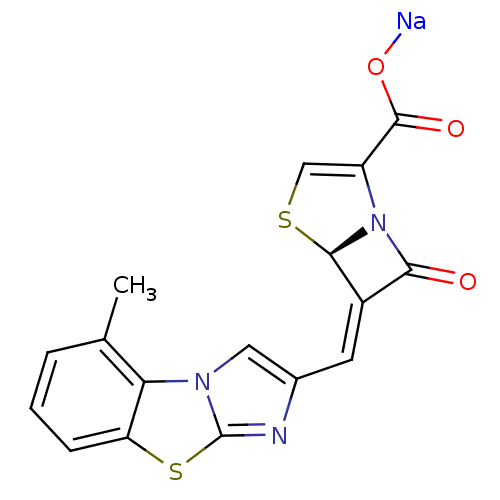 ((5R,6Z)-6-[(5-methylimidazo[2,1-b][1,3]-benzothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157691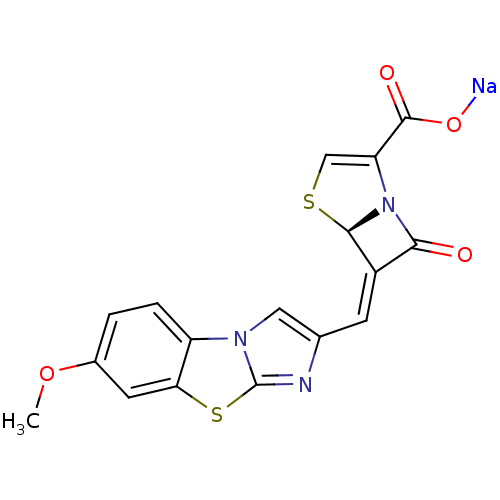 ((5R,6Z)-6-[(7-methoxyimidazo[2,1-b][1,3]-benzothia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50149467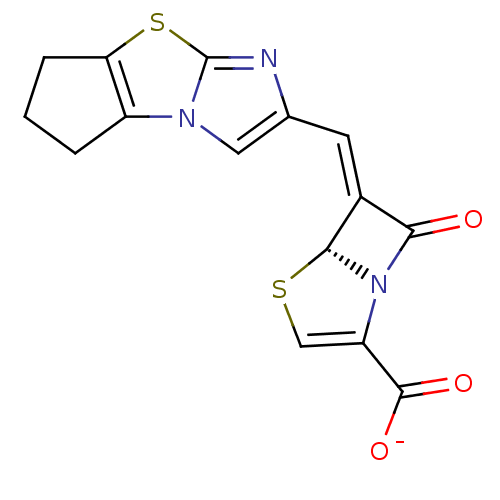 ((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149467 ((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149466 (CHEMBL124416 | Sodium; (R)-6-[1-(5,6-dihydro-4H-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of Escherichia coli CTX-M-15 | Antimicrob Agents Chemother 51: 3089-95 (2007) Article DOI: 10.1128/AAC.00218-07 BindingDB Entry DOI: 10.7270/Q2BG2NR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191390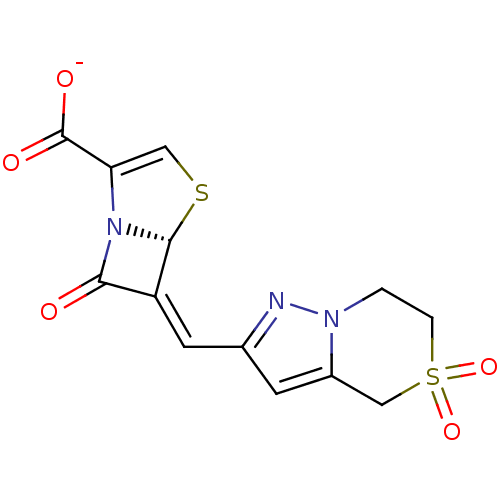 ((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191383 ((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466999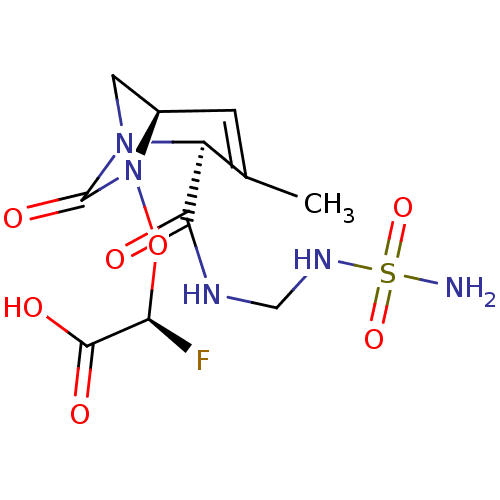 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[(sulfa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50114512 (CHEMBL45136 | Sodium; (R)-3-bromo-5,5,8-trioxo-7-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against Class C beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50114505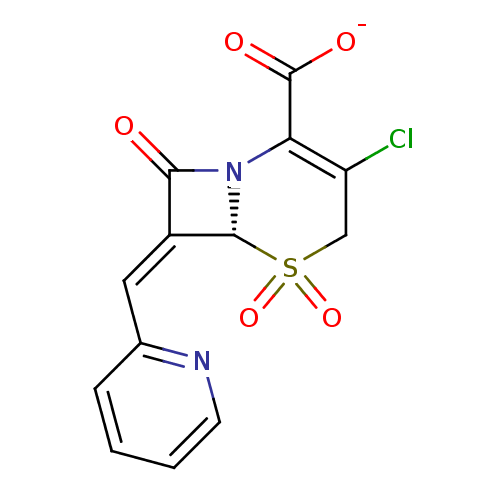 (CHEMBL295625 | Sodium; (R)-3-chloro-5,5,8-trioxo-7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against Class C beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191377 ((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191386 ((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157693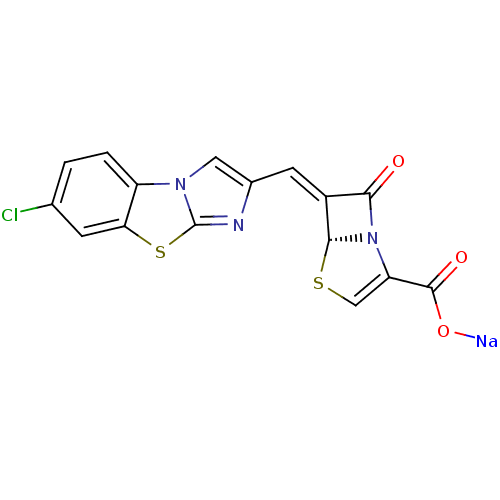 ((5R,6Z)-6-[(7-chloroimidazo[2,1-b][1,3]-benzothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466998 ((2S)-2-fluoro-2-[[(2S,5R)-2-(acetamidomethylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Class A beta-lactamase BCL-1 (Bacillus clausii) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
H�pital de Bic�tre Curated by ChEMBL | Assay Description Inhibition of Bacillus clausii NR beta-lactamase BCL1 expressed in Escherichia coli BL21 (DE3) | Antimicrob Agents Chemother 51: 4009-14 (2007) Article DOI: 10.1128/AAC.00537-07 BindingDB Entry DOI: 10.7270/Q20R9QBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695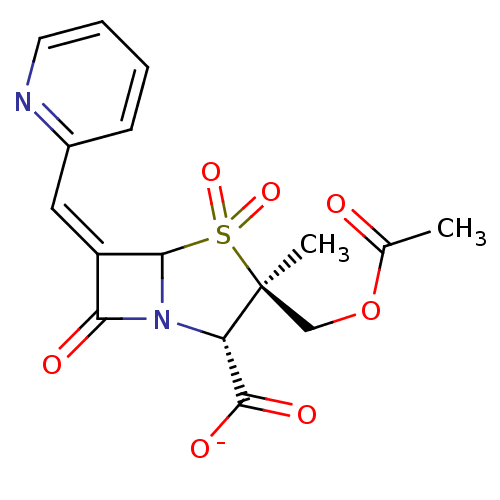 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191389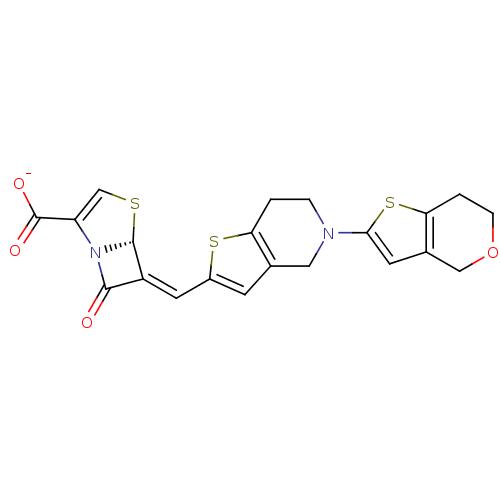 (6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50290040 (CHEMBL67925 | Potassium; (5R,6S)-3-tert-butyl-6-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for beta-lactamase inhibition activity of Enterobacter cloacae after 15 min of preincubation with the enzyme at 37 degree C | Bioorg Med Chem Lett 7: 2217-2222 (1997) Article DOI: 10.1016/S0960-894X(97)00401-0 BindingDB Entry DOI: 10.7270/Q2M32VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466979 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(2-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466987 ((2S)-2-[[(2S,5R)-2-[(3-amino-3-oxo-propyl)carbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50114504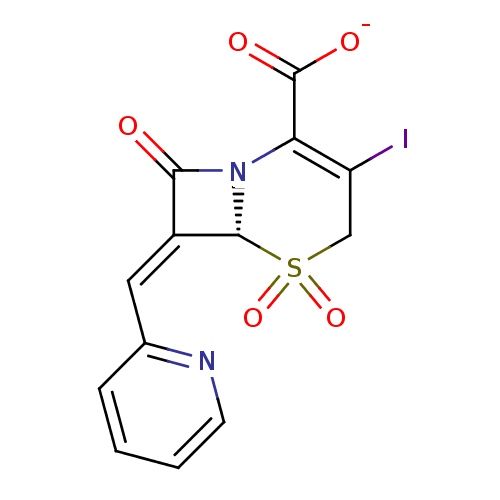 (CHEMBL45084 | Sodium; (R)-3-iodo-5,5,8-trioxo-7-[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against Class C beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149468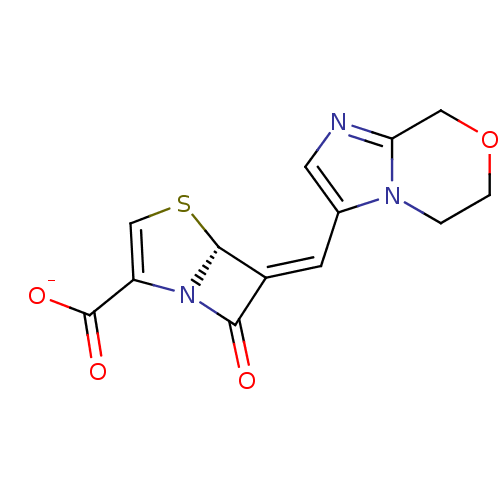 (CHEMBL263746 | Sodium; (R)-6-[1-(5,6-dihydro-8H-im...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191378 (CHEMBL212163 | sodium (R,E)-6-((6,8-dihydro-5H-imi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191388 ((5R)(6Z)-6-(6,7-5H-dihydropyrazolo[5,1-b]-oxazin-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347189 (CHEMBL1795572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466975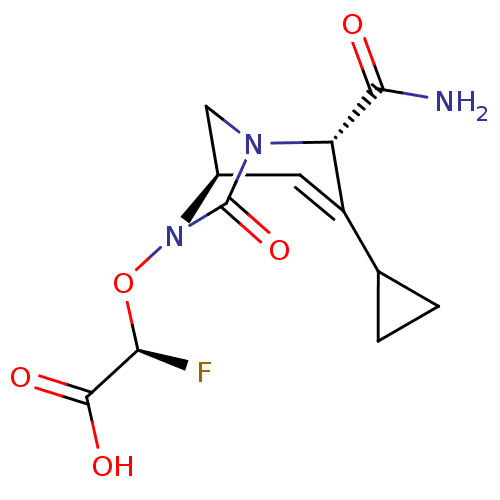 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50114514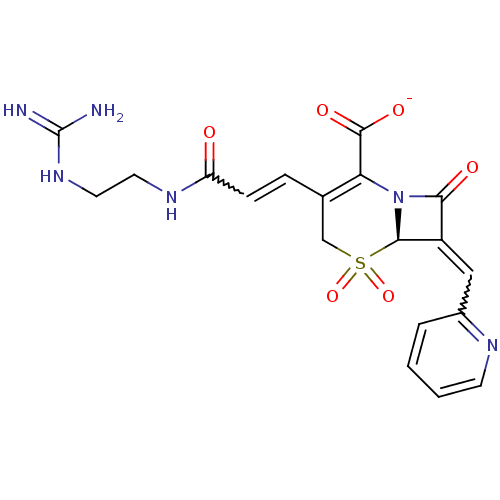 (CHEMBL44932 | Sodium; (R)-3-[(E)-2-(2-guanidino-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against Class C beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191385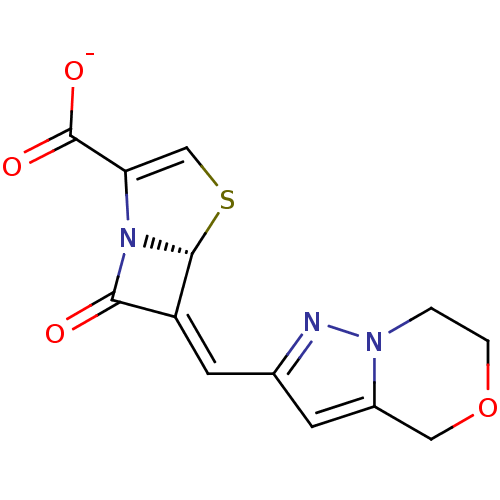 ((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191387 ((5R),(6Z)-6-(7-methyl-5,6,7,8-tetrahydroimidazo[1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50149469 (CHEMBL331090 | Sodium; (R)-7-oxo-6-[1-(5,6,7,8-tet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Class C (Amp-C) beta-Lactamases | J Med Chem 47: 3674-88 (2004) Article DOI: 10.1021/jm049903j BindingDB Entry DOI: 10.7270/Q2MK6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50191380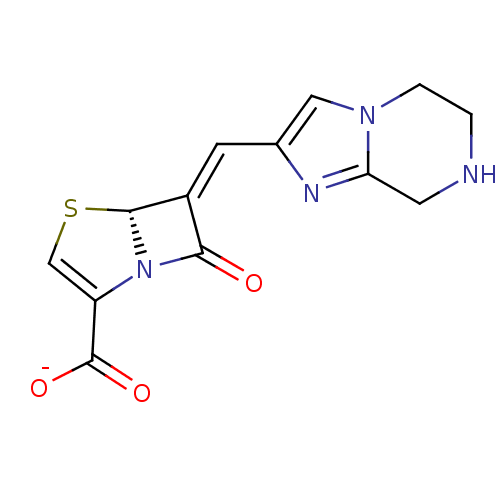 (CHEMBL379440 | sodium (R,E)-7-oxo-6-((5,6,7,8-tetr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 49: 4623-37 (2006) Article DOI: 10.1021/jm060021p BindingDB Entry DOI: 10.7270/Q2TX3F1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466974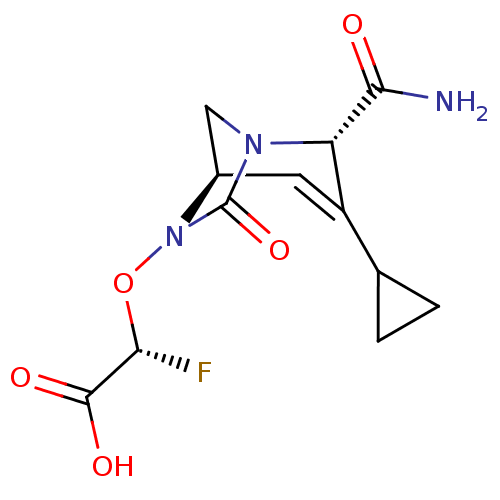 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466983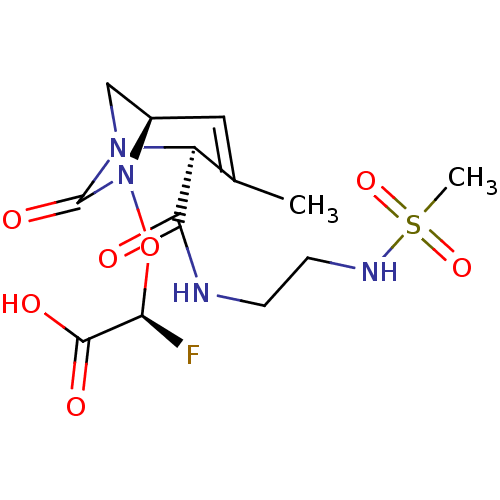 ((2S)-2-fluoro-2-[[(2S,5R)-2-[2-(methanesulfonamido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466977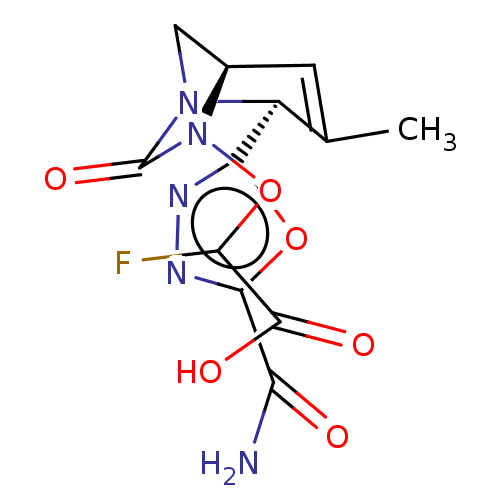 (2-[[(2S,5R)-2-(5-carbamoyl-1,3,4-oxadiazol-2-yl)-3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347184 (CHEMBL1795567) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114518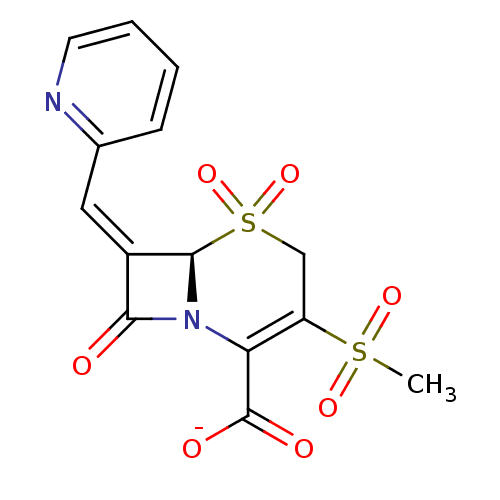 (CHEMBL297805 | Sodium; (R)-3-methanesulfonyl-5,5,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466970 (2-(((2S,5R)-2-(2-acetylhydrazinecarbonyl)-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114514 (CHEMBL44932 | Sodium; (R)-3-[(E)-2-(2-guanidino-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of Escherichia coli CTX-M-15 | Antimicrob Agents Chemother 51: 3089-95 (2007) Article DOI: 10.1128/AAC.00218-07 BindingDB Entry DOI: 10.7270/Q2BG2NR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466984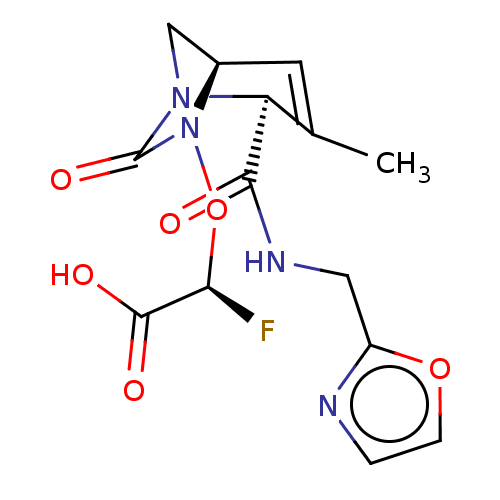 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-2-(oxazol-2-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50114516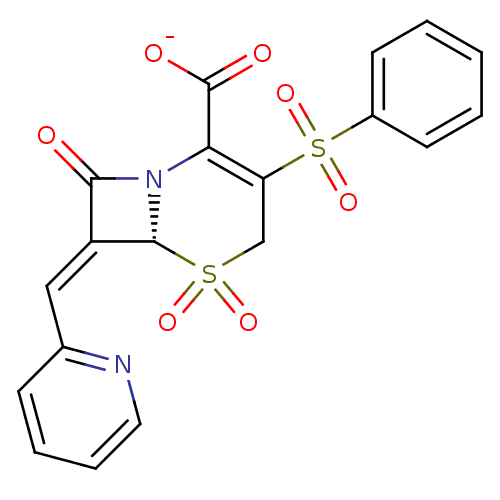 (CHEMBL295322 | Sodium; (R)-3-benzenesulfonyl-5,5,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against class A TEM-1 beta-lactamase | Bioorg Med Chem Lett 12: 1663-6 (2002) BindingDB Entry DOI: 10.7270/Q2MC8ZB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1026 total ) | Next | Last >> |