

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50222459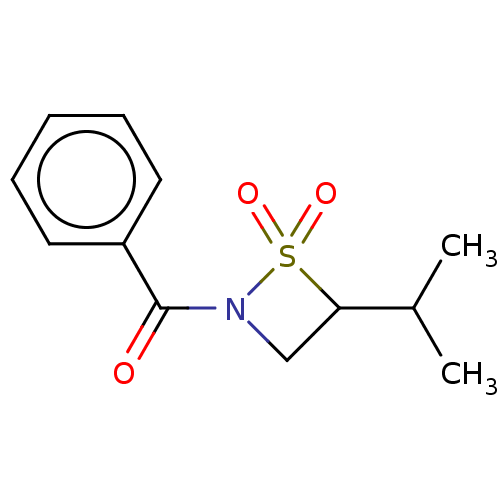 (CHEMBL140766) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.0700 | 7.0 | n/a |
The University of Huddersfield Curated by ChEMBL | Assay Description Inhibitor activity against Beta-lactamase, derived from the Gram negative bacteria Enterobacter cloacae at pH 7 | Bioorg Med Chem Lett 13: 4489-92 (2003) BindingDB Entry DOI: 10.7270/Q2FQ9ZTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067056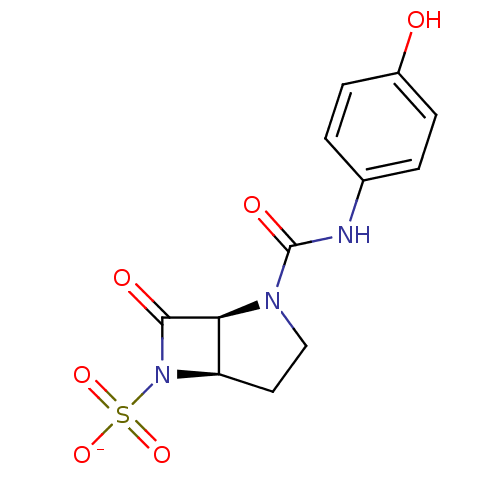 (CHEMBL338351 | Sodium; (1S,5R)-2-(4-hydroxy-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067062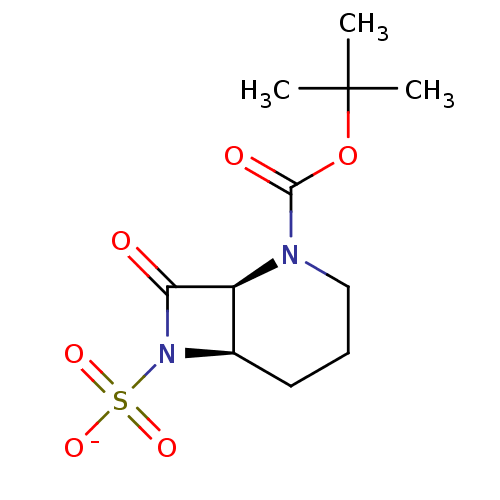 (CHEMBL415234 | Sodium; (1S,6R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067062 (CHEMBL415234 | Sodium; (1S,6R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.000700 | 0.5 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523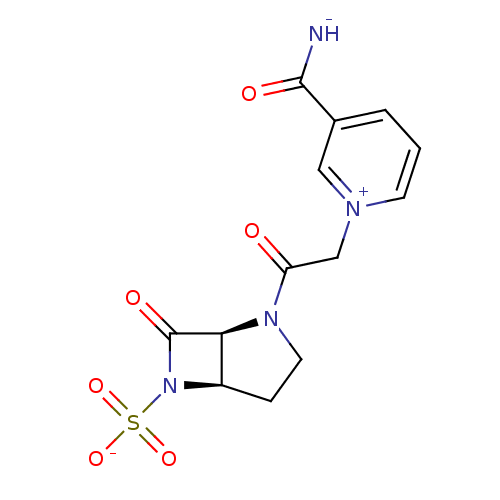 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.00000900 | 0.900 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067050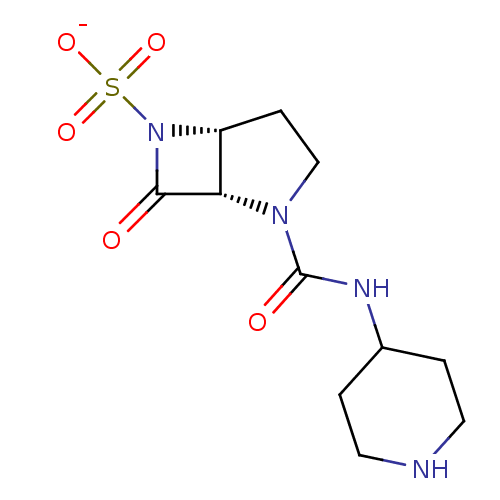 (CHEMBL336630 | Sodium; (1S,5R)-7-oxo-2-(piperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.00120 | 1.5 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067050 (CHEMBL336630 | Sodium; (1S,5R)-7-oxo-2-(piperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067065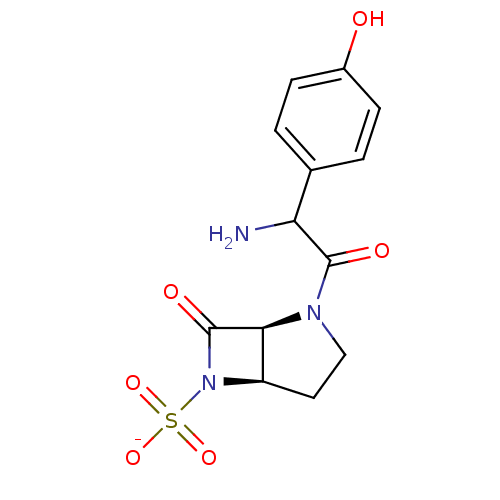 (CHEMBL130854 | Sodium; (1S,5R)-2-[2-amino-2-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067065 (CHEMBL130854 | Sodium; (1S,5R)-2-[2-amino-2-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | <0.0000100 | 2.70 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067066 (CHEMBL340707 | Sodium; (1S,4R,5S)-2-benzyloxycarbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.80 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067060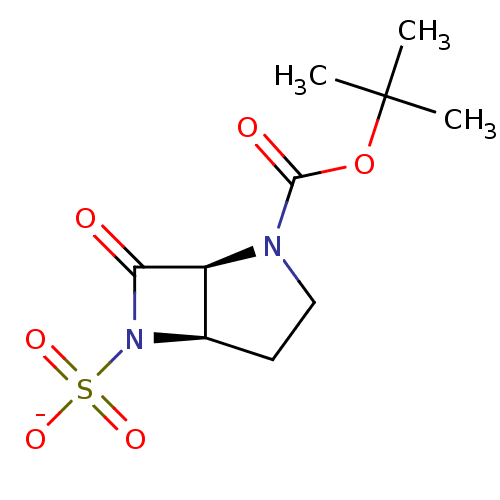 (CHEMBL128523 | Sodium; (1S,5R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.0000370 | 11 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067060 (CHEMBL128523 | Sodium; (1S,5R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 11 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537179 (CHEMBL4593963) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 20 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50222458 (CHEMBL341708) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 163 | 7.0 | n/a |
The University of Huddersfield Curated by ChEMBL | Assay Description Inhibitor activity against Beta-lactamase, derived from the Gram negative bacteria Enterobacter cloacae at pH 7 | Bioorg Med Chem Lett 13: 4489-92 (2003) BindingDB Entry DOI: 10.7270/Q2FQ9ZTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537175 (CHEMBL4520276) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 180 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537177 (CHEMBL4583358) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537174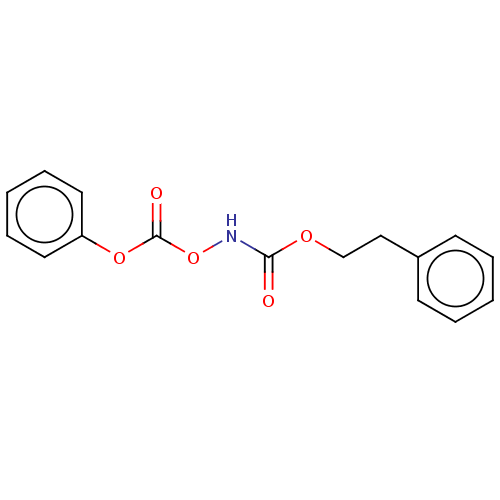 (CHEMBL4514634) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537173 (CHEMBL4582529) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537178 (CHEMBL4594060) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 8.50E+3 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50537176 (CHEMBL4535968) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Irreversible inhibition of Enterobacter cloacae P99 beta-lactamase using cephalothin as substrate by spectrophotometric analysis | Bioorg Med Chem 27: 1430-1436 (2019) Article DOI: 10.1016/j.bmc.2019.02.023 BindingDB Entry DOI: 10.7270/Q2MK6HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||