 Found 833 hits of ic50 data for polymerid = 4416
Found 833 hits of ic50 data for polymerid = 4416 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578
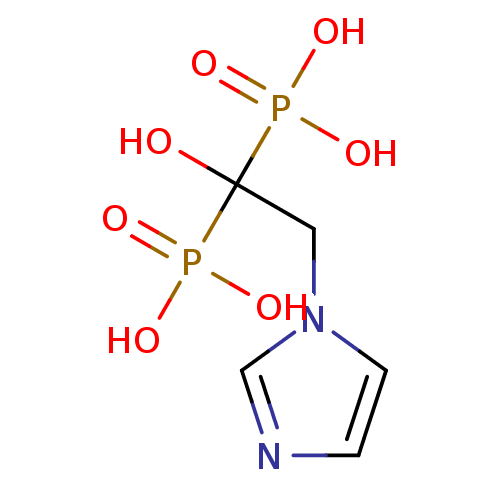
(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576
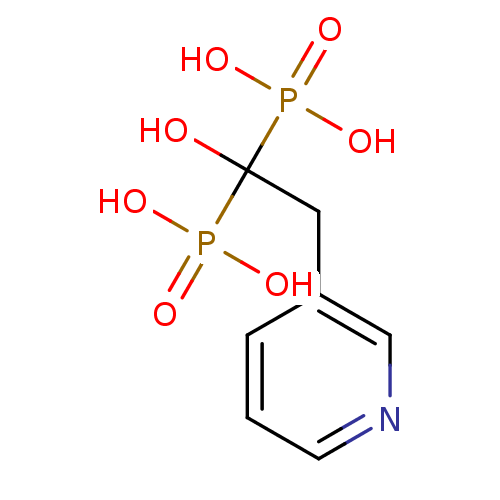
(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS using pre-incubation of compound with enzyme |
ACS Med Chem Lett 6: 349-54 (2015)
Article DOI: 10.1021/ml500528x
BindingDB Entry DOI: 10.7270/Q2251KW1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422472
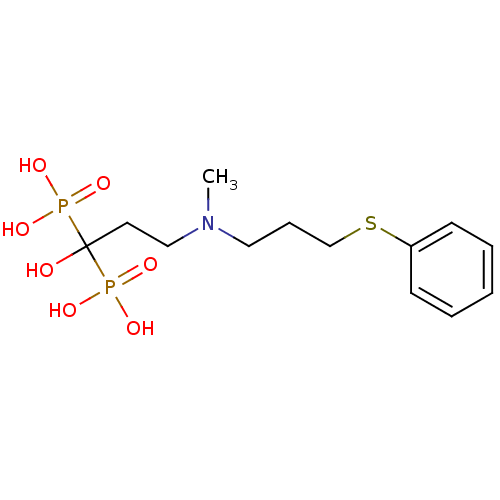
(CHEMBL100827)Show InChI InChI=1S/C13H23NO7P2S/c1-14(9-5-11-24-12-6-3-2-4-7-12)10-8-13(15,22(16,17)18)23(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25290
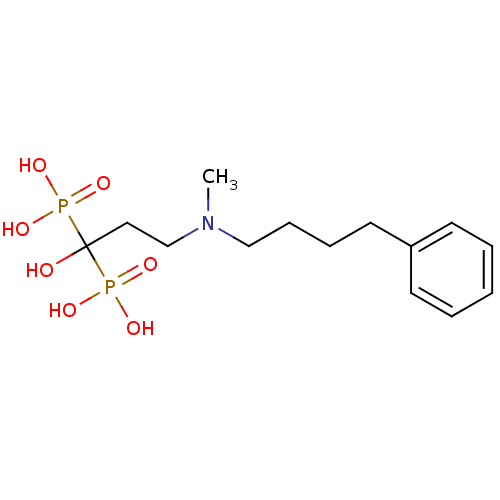
(CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...)Show InChI InChI=1S/C14H25NO7P2/c1-15(11-6-5-9-13-7-3-2-4-8-13)12-10-14(16,23(17,18)19)24(20,21)22/h2-4,7-8,16H,5-6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098389
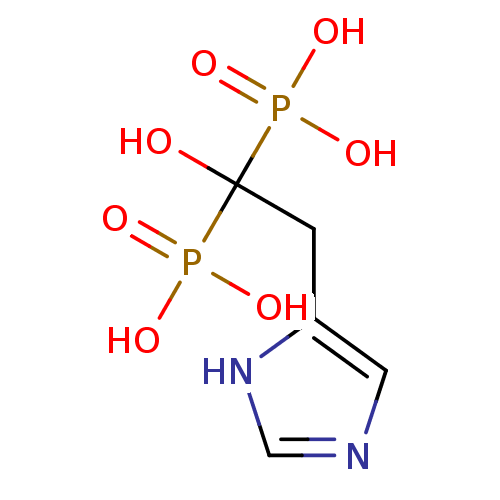
(1-hydroxy-2-(1H-imidazol-5-yl)ethane-1,1-diyldipho...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)1-4-2-6-3-7-4/h2-3,8H,1H2,(H,6,7)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422449

(CHEMBL101886)Show InChI InChI=1S/C13H23NO8P2/c1-14(9-5-11-22-12-6-3-2-4-7-12)10-8-13(15,23(16,17)18)24(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422469
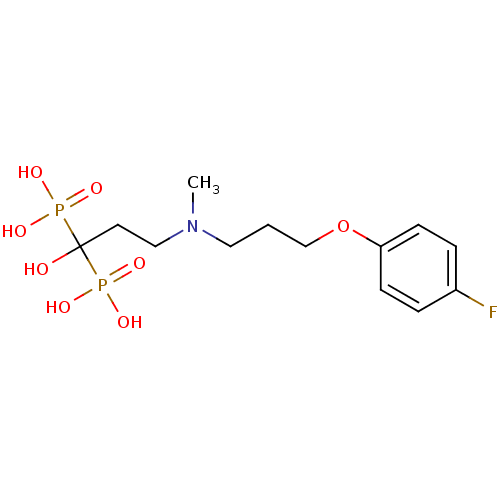
(CHEMBL101472)Show SMILES CN(CCCOc1ccc(F)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22FNO8P2/c1-15(8-2-10-23-12-5-3-11(14)4-6-12)9-7-13(16,24(17,18)19)25(20,21)22/h3-6,16H,2,7-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422457
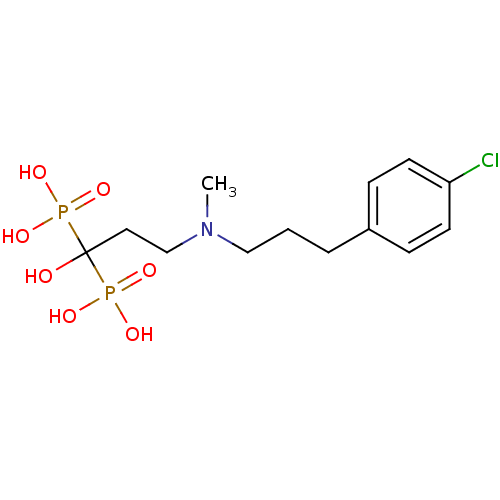
(CHEMBL101230)Show SMILES CN(CCCc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22ClNO7P2/c1-15(9-2-3-11-4-6-12(14)7-5-11)10-8-13(16,23(17,18)19)24(20,21)22/h4-7,16H,2-3,8-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422470
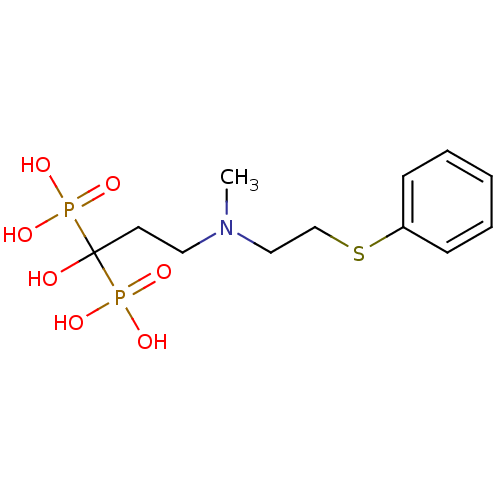
(CHEMBL100508)Show InChI InChI=1S/C12H21NO7P2S/c1-13(9-10-23-11-5-3-2-4-6-11)8-7-12(14,21(15,16)17)22(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25308
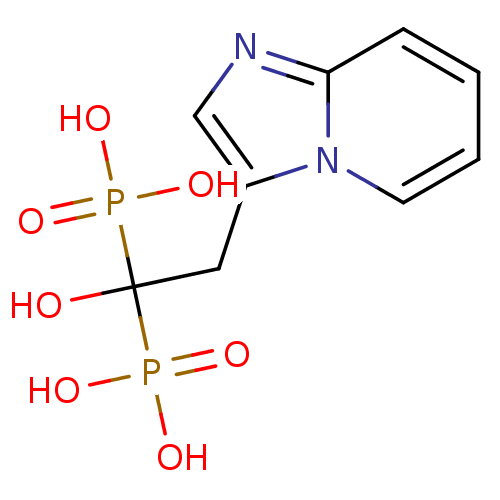
((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 25: 1117-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.089
BindingDB Entry DOI: 10.7270/Q25X2BMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422463
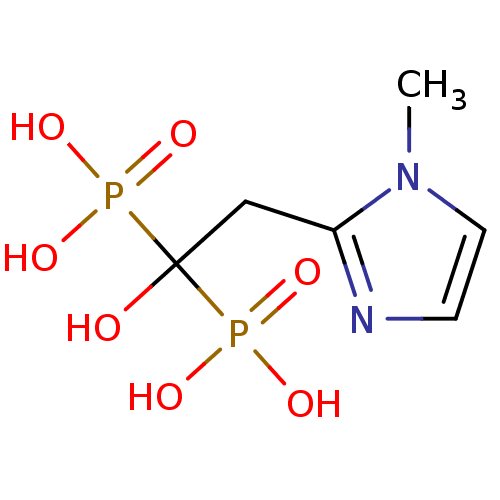
(CHEMBL101207)Show InChI InChI=1S/C6H12N2O7P2/c1-8-3-2-7-5(8)4-6(9,16(10,11)12)17(13,14)15/h2-3,9H,4H2,1H3,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human FPPS (1 to 353 residues) expressed in Escherichia coli BL21 (DE3) pre-incubated for 10 mins before addition... |
J Med Chem 62: 9691-9702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01104
BindingDB Entry DOI: 10.7270/Q23T9MJV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50117257
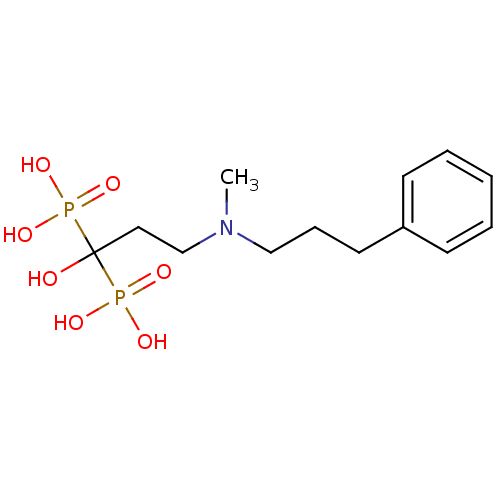
(1-hydroxy-3-(methyl(3-phenylpropyl)amino)propane-1...)Show InChI InChI=1S/C13H23NO7P2/c1-14(10-5-8-12-6-3-2-4-7-12)11-9-13(15,22(16,17)18)23(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135839
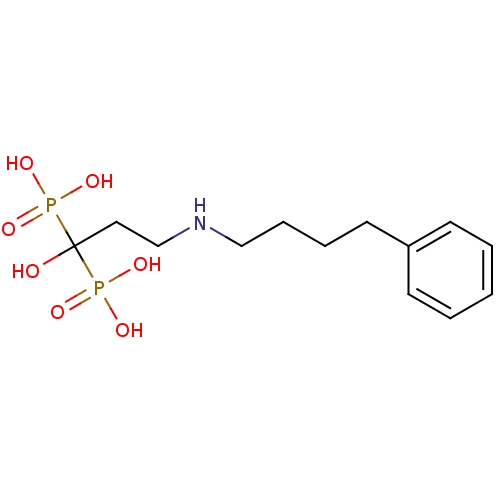
(CHEMBL55464 | [1-Hydroxy-3-(4-phenyl-butylamino)-1...)Show InChI InChI=1S/C13H23NO7P2/c15-13(22(16,17)18,23(19,20)21)9-11-14-10-5-4-8-12-6-2-1-3-7-12/h1-3,6-7,14-15H,4-5,8-11H2,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422455
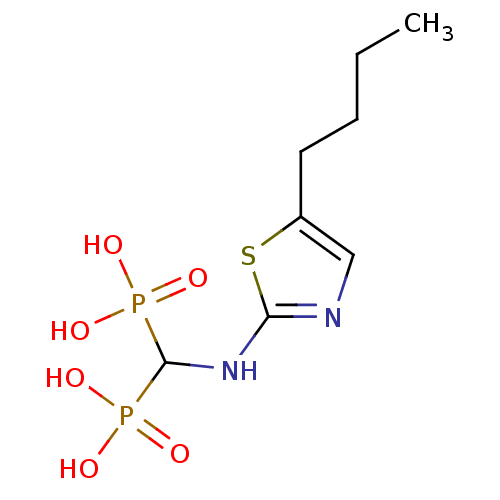
(CHEMBL98476)Show InChI InChI=1S/C8H16N2O6P2S/c1-2-3-4-6-5-9-7(19-6)10-8(17(11,12)13)18(14,15)16/h5,8H,2-4H2,1H3,(H,9,10)(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422454
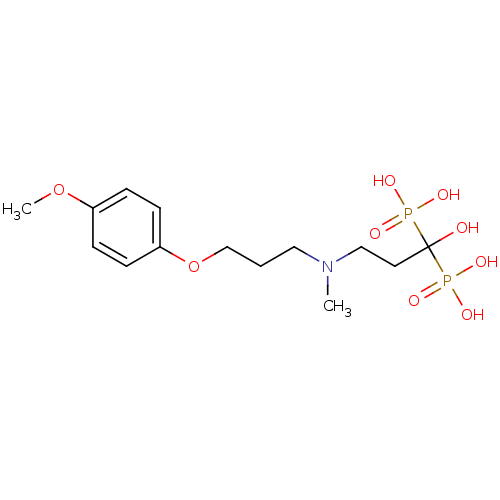
(CHEMBL316913)Show SMILES COc1ccc(OCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O)cc1 Show InChI InChI=1S/C14H25NO9P2/c1-15(10-8-14(16,25(17,18)19)26(20,21)22)9-3-11-24-13-6-4-12(23-2)5-7-13/h4-7,16H,3,8-11H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25308

((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of FDPS (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126757
BindingDB Entry DOI: 10.7270/Q2JM2DW5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of FDPS (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126757
BindingDB Entry DOI: 10.7270/Q2JM2DW5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25308

((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS after 10 mins using [14C]IPP as substrate by liquid scintillation counting |
J Med Chem 53: 3454-64 (2010)
Checked by Author
Article DOI: 10.1021/jm900232u
BindingDB Entry DOI: 10.7270/Q21837FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422446
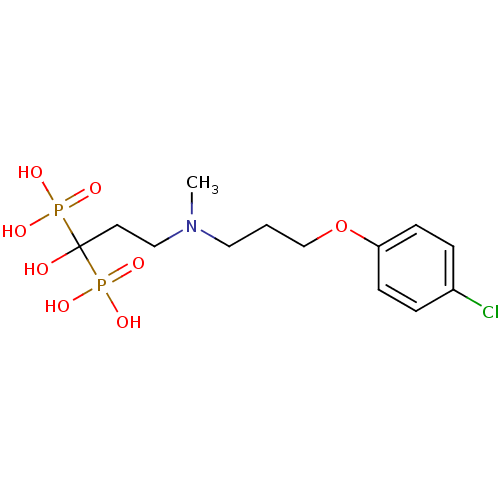
(CHEMBL101407)Show SMILES CN(CCCOc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22ClNO8P2/c1-15(8-2-10-23-12-5-3-11(14)4-6-12)9-7-13(16,24(17,18)19)25(20,21)22/h3-6,16H,2,7-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135831
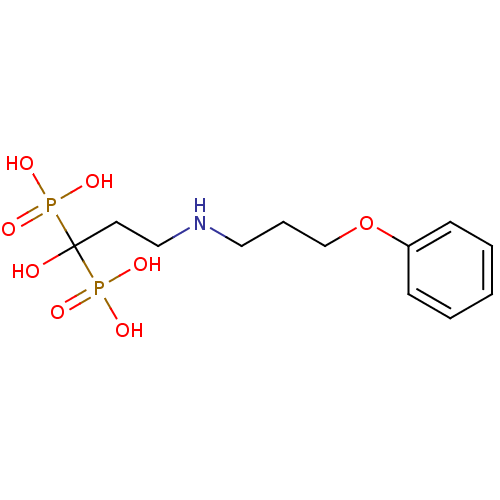
(CHEMBL316844 | [1-Hydroxy-3-(3-phenoxy-propylamino...)Show InChI InChI=1S/C12H21NO8P2/c14-12(22(15,16)17,23(18,19)20)7-9-13-8-4-10-21-11-5-2-1-3-6-11/h1-3,5-6,13-14H,4,7-10H2,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50117260
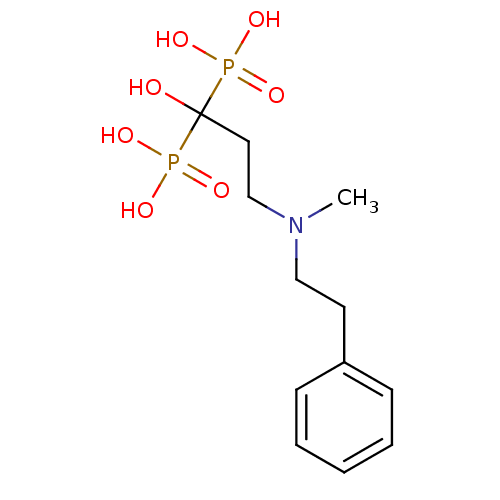
(1-hydroxy-3-(methyl(phenethyl)amino)propane-1,1-di...)Show InChI InChI=1S/C12H21NO7P2/c1-13(9-7-11-5-3-2-4-6-11)10-8-12(14,21(15,16)17)22(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Cardioselectivity for the beta-1 adrenergic receptor was determined against isoprenaline (antagonism) in isolated rat atria |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02221 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422448
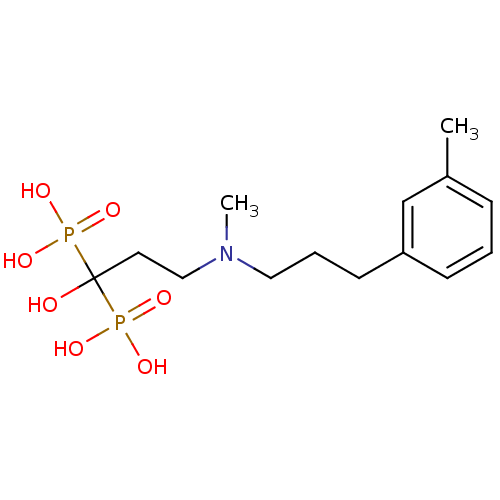
(CHEMBL100441)Show SMILES CN(CCCc1cccc(C)c1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO7P2/c1-12-5-3-6-13(11-12)7-4-9-15(2)10-8-14(16,23(17,18)19)24(20,21)22/h3,5-6,11,16H,4,7-10H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098378
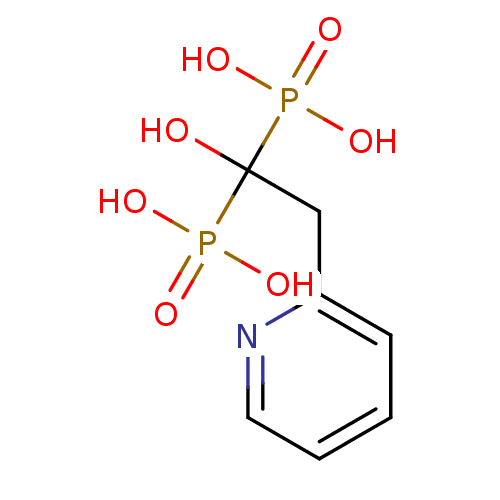
((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h1-4,9H,5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098378

((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h1-4,9H,5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FPPS expressed in Escherichia coli BL21 (DE3) preincubated for 10 mins in presence compound relative to control |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01913
BindingDB Entry DOI: 10.7270/Q28K7F00 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138041
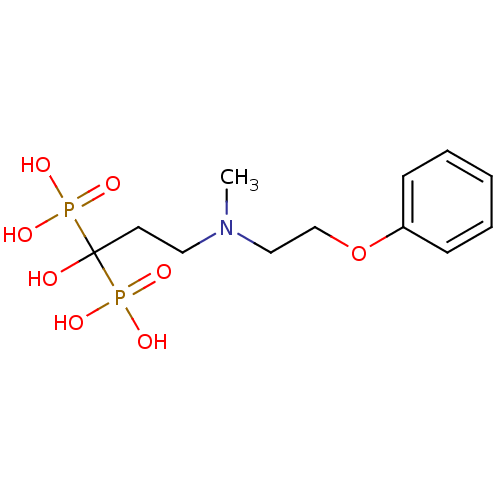
(1-hydroxy-3-(methyl(2-phenoxyethyl)amino)propane-1...)Show InChI InChI=1S/C12H21NO8P2/c1-13(9-10-21-11-5-3-2-4-6-11)8-7-12(14,22(15,16)17)23(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
| Assay Description
To measure the kinetic constants by radioactive assays, 0.2 μM wild-type E. coli OPPS and the wild-type or mutant S. cerevisiae GGPPS and human ... |
Biochemistry 55: 4366-74 (2016)
Article DOI: 10.1021/acs.biochem.6b00486
BindingDB Entry DOI: 10.7270/Q2BR8QZK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422464
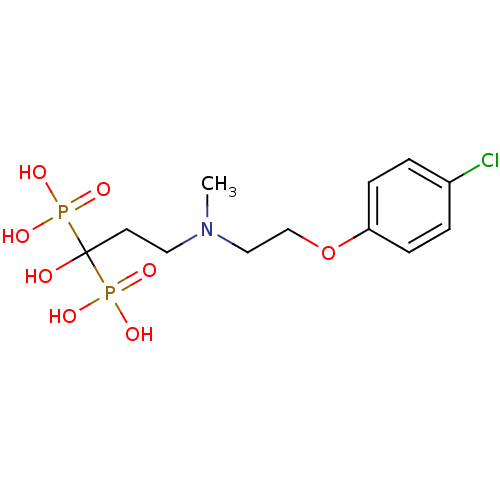
(CHEMBL319519)Show SMILES CN(CCOc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C12H20ClNO8P2/c1-14(8-9-22-11-4-2-10(13)3-5-11)7-6-12(15,23(16,17)18)24(19,20)21/h2-5,15H,6-9H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [3H]IPP and GPP as substrate incubated for 10 mi... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of human FPP synthase expressed in Escherichia coli BL21 (DE3) |
J Med Chem 51: 6800-7 (2008)
Article DOI: 10.1021/jm801003y
BindingDB Entry DOI: 10.7270/Q2F76CD8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 25: 1117-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.089
BindingDB Entry DOI: 10.7270/Q25X2BMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Cardioselectivity for the beta-1 adrenergic receptor was determined against isoprenaline (antagonism) in isolated guinea pig trachea |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02221 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422458
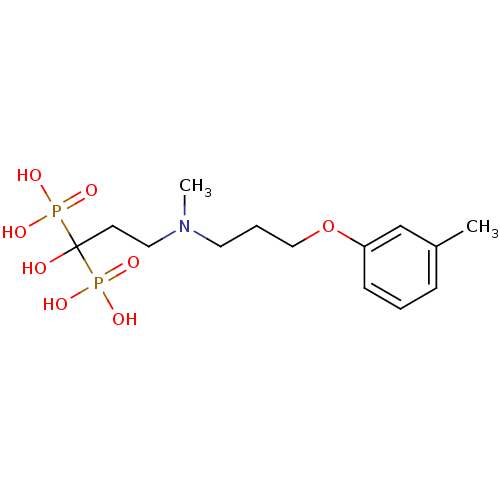
(CHEMBL100835)Show SMILES CN(CCCOc1cccc(C)c1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO8P2/c1-12-5-3-6-13(11-12)23-10-4-8-15(2)9-7-14(16,24(17,18)19)25(20,21)22/h3,5-6,11,16H,4,7-10H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422467
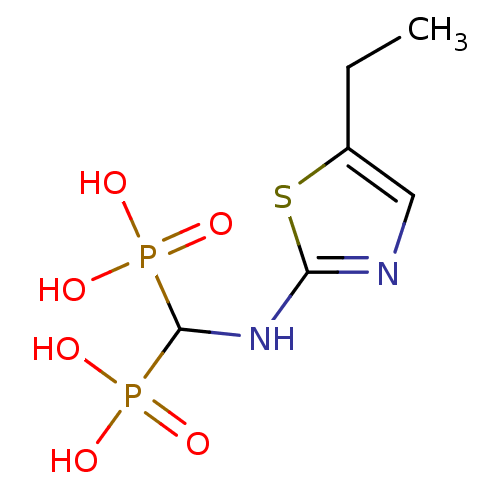
(CHEMBL100830)Show InChI InChI=1S/C6H12N2O6P2S/c1-2-4-3-7-5(17-4)8-6(15(9,10)11)16(12,13)14/h3,6H,2H2,1H3,(H,7,8)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422475
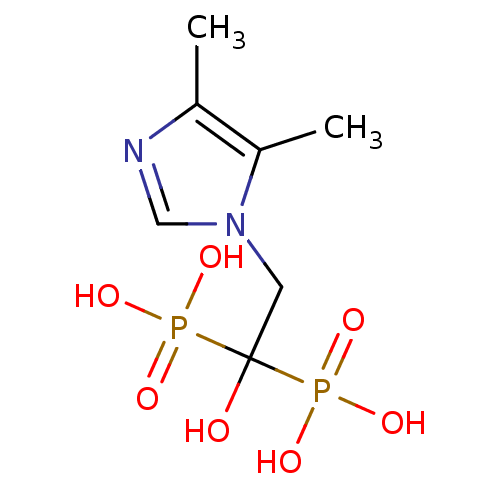
(CHEMBL98703)Show InChI InChI=1S/C7H14N2O7P2/c1-5-6(2)9(4-8-5)3-7(10,17(11,12)13)18(14,15)16/h4,10H,3H2,1-2H3,(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422461
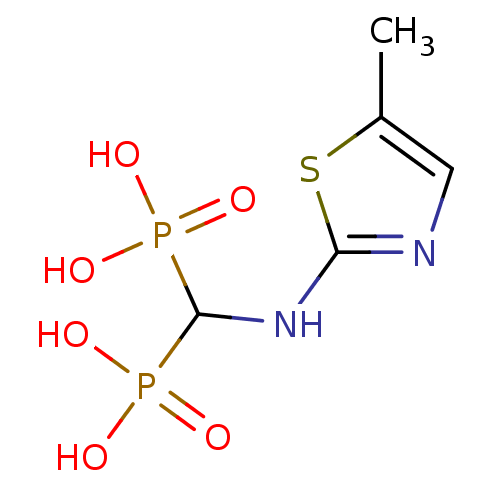
(CHEMBL100335)Show InChI InChI=1S/C5H10N2O6P2S/c1-3-2-6-4(16-3)7-5(14(8,9)10)15(11,12)13/h2,5H,1H3,(H,6,7)(H2,8,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422477
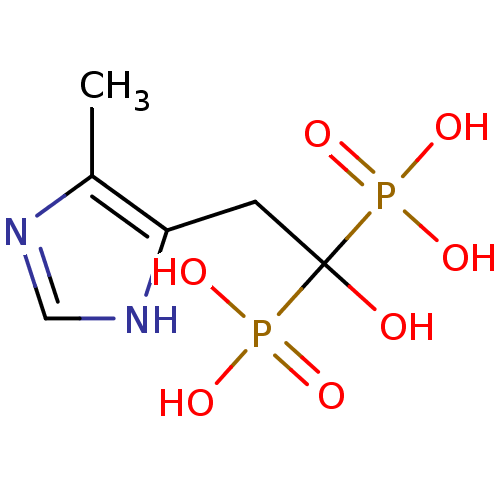
(CHEMBL317646)Show InChI InChI=1S/C6H12N2O7P2/c1-4-5(8-3-7-4)2-6(9,16(10,11)12)17(13,14)15/h3,9H,2H2,1H3,(H,7,8)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 18: 2878-82 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.088
BindingDB Entry DOI: 10.7270/Q2WM1F9P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of FDPS (unknown origin) |
Bioorg Med Chem Lett 25: 2331-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.021
BindingDB Entry DOI: 10.7270/Q2M61MZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098390
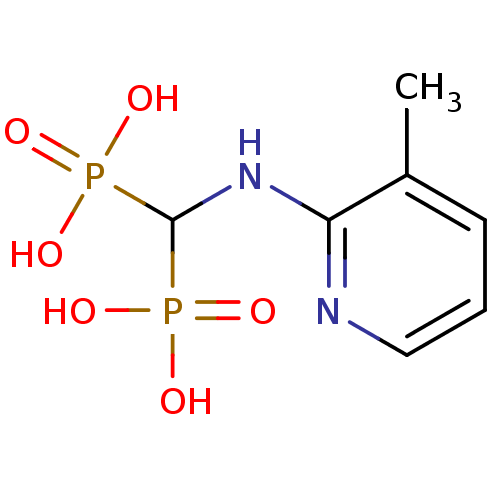
((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422444

(CHEMBL100295)Show InChI InChI=1S/C7H14N2O6P2S/c1-2-3-5-4-8-6(18-5)9-7(16(10,11)12)17(13,14)15/h4,7H,2-3H2,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422441
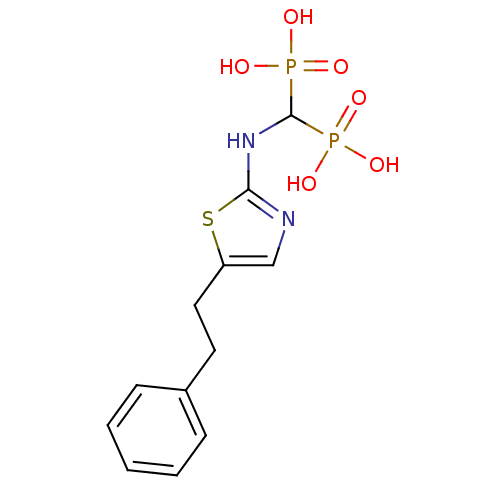
(CHEMBL101539)Show InChI InChI=1S/C12H16N2O6P2S/c15-21(16,17)12(22(18,19)20)14-11-13-8-10(23-11)7-6-9-4-2-1-3-5-9/h1-5,8,12H,6-7H2,(H,13,14)(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50273714
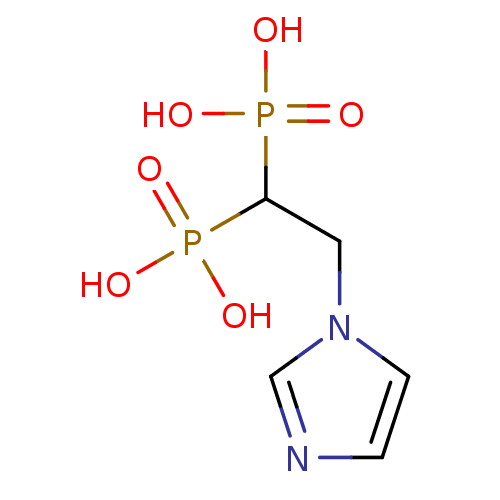
(CHEMBL446734 | [2-(Imidazol-1-yl)ethyl]-bisphospho...)Show InChI InChI=1S/C5H10N2O6P2/c8-14(9,10)5(15(11,12)13)3-7-2-1-6-4-7/h1-2,4-5H,3H2,(H2,8,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of human FPP synthase expressed in Escherichia coli BL21 (DE3) |
J Med Chem 51: 6800-7 (2008)
Article DOI: 10.1021/jm801003y
BindingDB Entry DOI: 10.7270/Q2F76CD8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data