 Found 42 hits of ic50 data for polymerid = 4420
Found 42 hits of ic50 data for polymerid = 4420 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232534
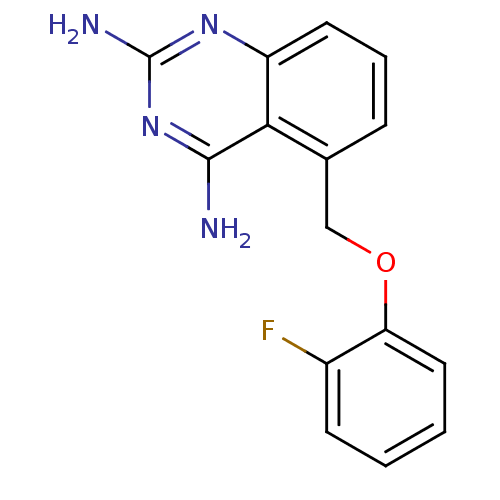
(5-((2-fluorophenoxy)methyl)quinazoline-2,4-diamine...)Show InChI InChI=1S/C15H13FN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50512855

(CHEMBL4465809)Show InChI InChI=1S/C15H13FN4O3S/c16-24(21,22)10-6-4-9(5-7-10)8-23-12-3-1-2-11-13(12)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of DcpS (unknown origin) using m7GpppA as substrate by ELISA |
J Med Chem 62: 5673-5724 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01153
BindingDB Entry DOI: 10.7270/Q2959MWN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232526
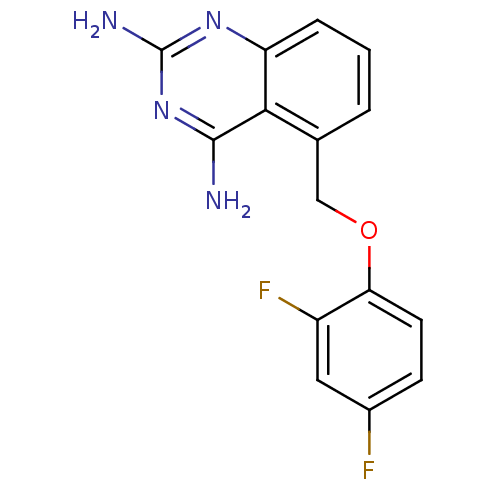
(5-((2,4-difluorophenoxy)methyl)quinazoline-2,4-dia...)Show InChI InChI=1S/C15H12F2N4O/c16-9-4-5-12(10(17)6-9)22-7-8-2-1-3-11-13(8)14(18)21-15(19)20-11/h1-6H,7H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232538
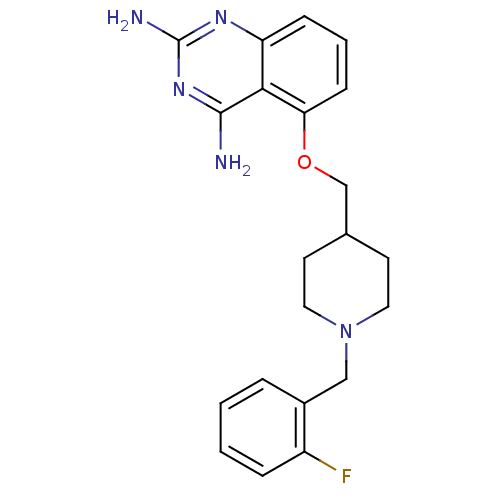
(5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...)Show InChI InChI=1S/C21H24FN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237216
(CHEMBL4080254)Show InChI InChI=1S/C21H25N5O/c22-20-19-17(24-21(23)25-20)7-4-8-18(19)27-14-16-9-11-26(12-10-16)13-15-5-2-1-3-6-15/h1-8,16H,9-14H2,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237201
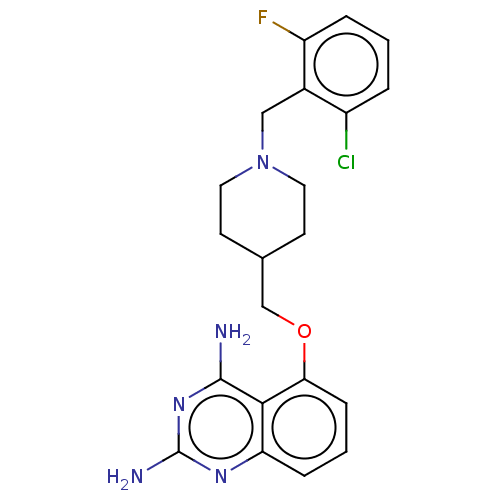
(CHEMBL4082618)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(F)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23ClFN5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232589
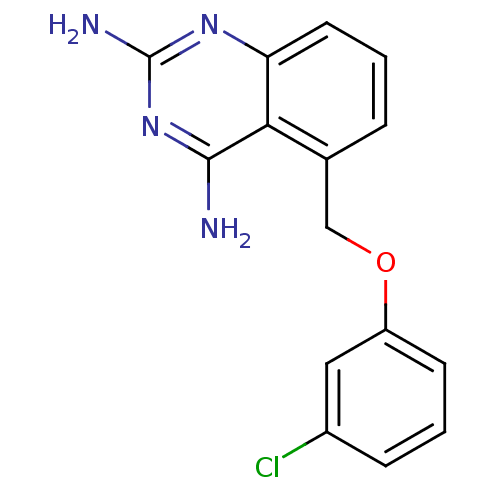
(5-((3-chlorophenoxy)methyl)quinazoline-2,4-diamine...)Show InChI InChI=1S/C15H13ClN4O/c16-10-4-2-5-11(7-10)21-8-9-3-1-6-12-13(9)14(17)20-15(18)19-12/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237200
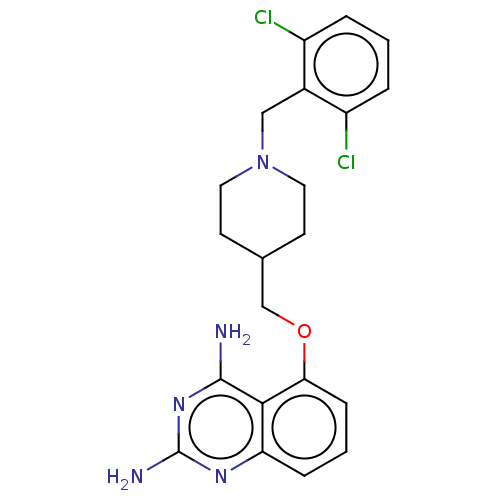
(CHEMBL4072132)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(Cl)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36530
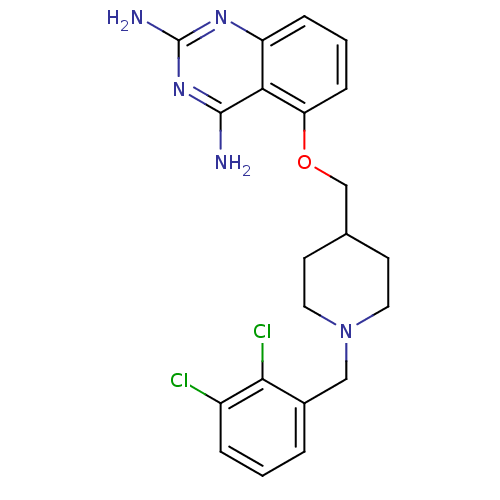
(D157493)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4cccc(Cl)c4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-4-1-3-14(19(15)23)11-28-9-7-13(8-10-28)12-29-17-6-2-5-16-18(17)20(24)27-21(25)26-16/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-HT3 receptor in rat was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237203
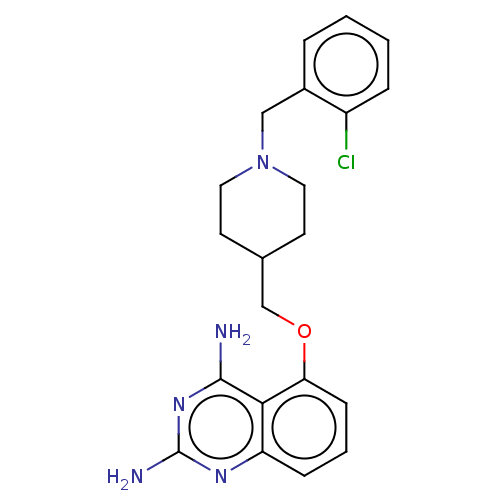
(CHEMBL250072)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H24ClN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237199
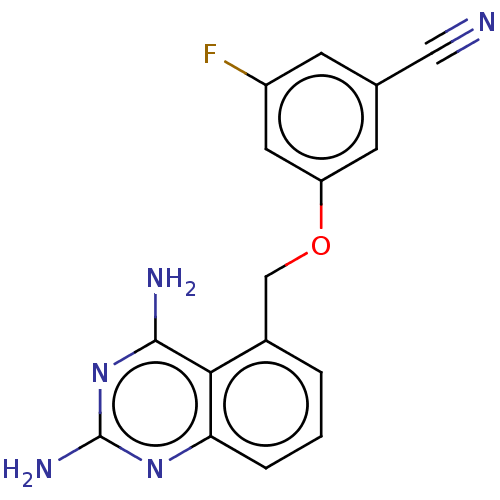
(CHEMBL4077061)Show InChI InChI=1S/C16H12FN5O/c17-11-4-9(7-18)5-12(6-11)23-8-10-2-1-3-13-14(10)15(19)22-16(20)21-13/h1-6H,8H2,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237205
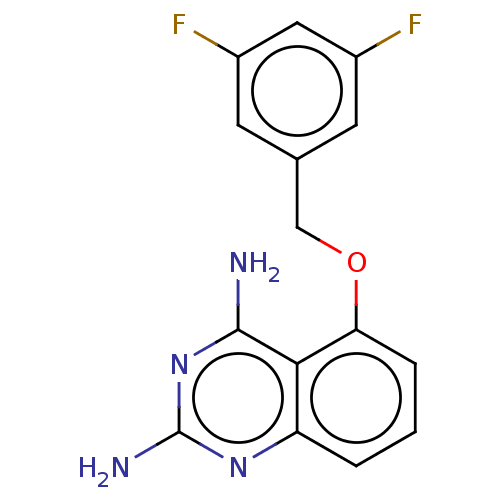
(CHEMBL398675)Show InChI InChI=1S/C15H12F2N4O/c16-9-4-8(5-10(17)6-9)7-22-12-3-1-2-11-13(12)14(18)21-15(19)20-11/h1-6H,7H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237209
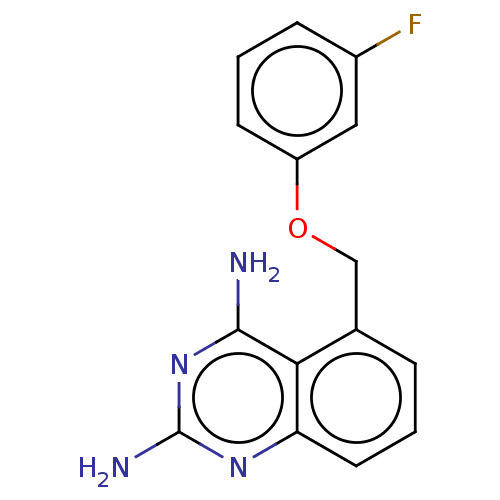
(CHEMBL4062544)Show InChI InChI=1S/C15H13FN4O/c16-10-4-2-5-11(7-10)21-8-9-3-1-6-12-13(9)14(17)20-15(18)19-12/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237211
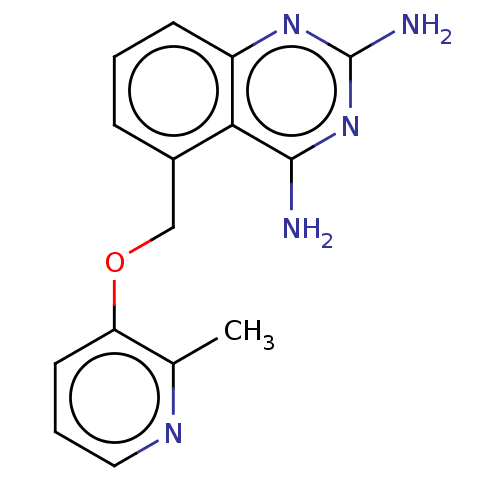
(CHEMBL4061457)Show InChI InChI=1S/C15H15N5O/c1-9-12(6-3-7-18-9)21-8-10-4-2-5-11-13(10)14(16)20-15(17)19-11/h2-7H,8H2,1H3,(H4,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237210
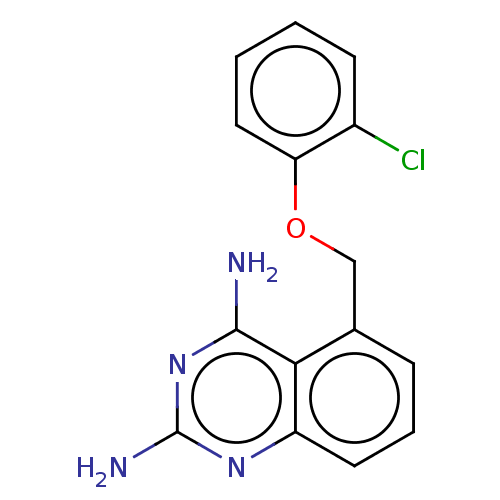
(CHEMBL399673)Show InChI InChI=1S/C15H13ClN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36534
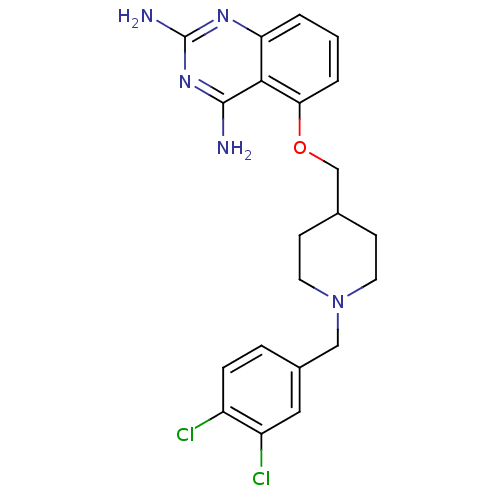
(D156095)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccc(Cl)c(Cl)c4)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-5-4-14(10-16(15)23)11-28-8-6-13(7-9-28)12-29-18-3-1-2-17-19(18)20(24)27-21(25)26-17/h1-5,10,13H,6-9,11-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237217
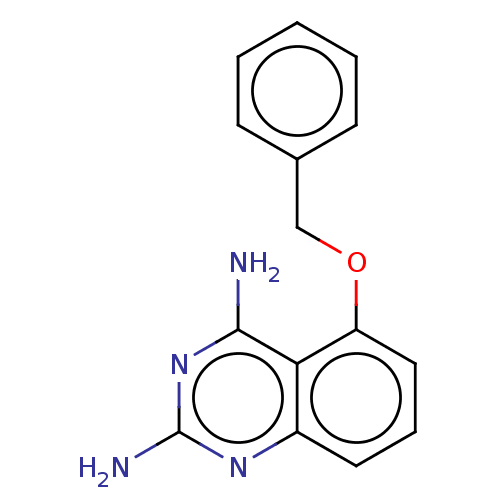
(CHEMBL342595)Show InChI InChI=1S/C15H14N4O/c16-14-13-11(18-15(17)19-14)7-4-8-12(13)20-9-10-5-2-1-3-6-10/h1-8H,9H2,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards rat 5-hydroxytryptamine 3 receptor was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237202
(CHEMBL4103454)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccccc4OC(F)(F)F)CC3)cccc2n1 Show InChI InChI=1S/C22H24F3N5O2/c23-22(24,25)32-17-6-2-1-4-15(17)12-30-10-8-14(9-11-30)13-31-18-7-3-5-16-19(18)20(26)29-21(27)28-16/h1-7,14H,8-13H2,(H4,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237198
(CHEMBL4068466)Show InChI InChI=1S/C14H18N4O2/c15-13-12-10(17-14(16)18-13)2-1-3-11(12)20-8-9-4-6-19-7-5-9/h1-3,9H,4-8H2,(H4,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237218

(CHEMBL4072481)Show InChI InChI=1S/C14H18N4O2/c15-13-12-9(8-20-10-4-6-19-7-5-10)2-1-3-11(12)17-14(16)18-13/h1-3,10H,4-8H2,(H4,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232566
(5-(3-chlorobenzyloxy)quinazoline-2,4-diamine | CHE...)Show InChI InChI=1S/C15H13ClN4O/c16-10-4-1-3-9(7-10)8-21-12-6-2-5-11-13(12)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237217

(CHEMBL342595)Show InChI InChI=1S/C15H14N4O/c16-14-13-11(18-15(17)19-14)7-4-8-12(13)20-9-10-5-2-1-3-6-10/h1-8H,9H2,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of DcpS (unknown origin) using m7GpppA as substrate by ELISA |
J Med Chem 62: 5673-5724 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01153
BindingDB Entry DOI: 10.7270/Q2959MWN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237200

(CHEMBL4072132)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(Cl)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232538

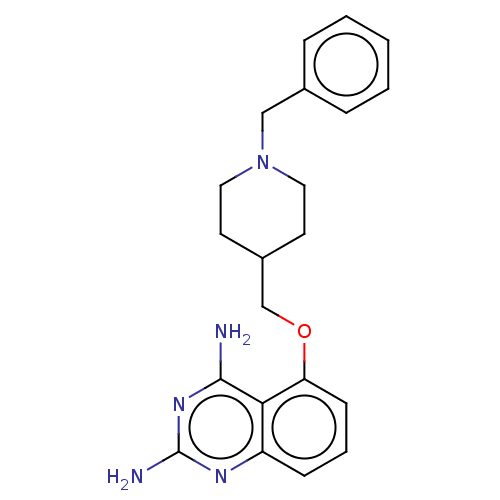
(5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...)Show InChI InChI=1S/C21H24FN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 7.62 | n/a | 4 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237204

(CHEMBL343543)Show InChI InChI=1S/C9H10N4O/c1-14-6-4-2-3-5-7(6)8(10)13-9(11)12-5/h2-4H,1H3,(H4,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36533
(D157555)Show InChI InChI=1S/C19H25N7O/c1-25-10-7-22-16(25)11-26-8-5-13(6-9-26)12-27-15-4-2-3-14-17(15)18(20)24-19(21)23-14/h2-4,7,10,13H,5-6,8-9,11-12H2,1H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.38 | n/a | 108 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237206
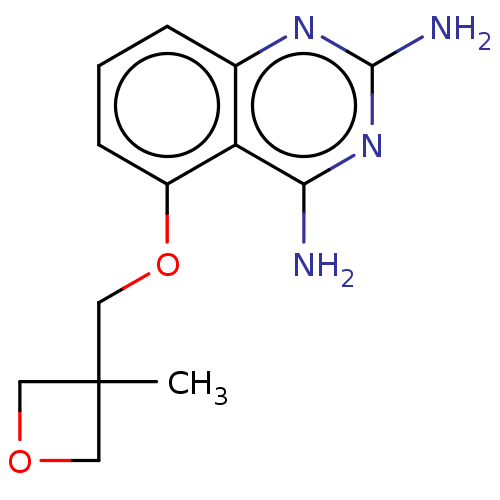
(CHEMBL4075292)Show InChI InChI=1S/C13H16N4O2/c1-13(5-18-6-13)7-19-9-4-2-3-8-10(9)11(14)17-12(15)16-8/h2-4H,5-7H2,1H3,(H4,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237212
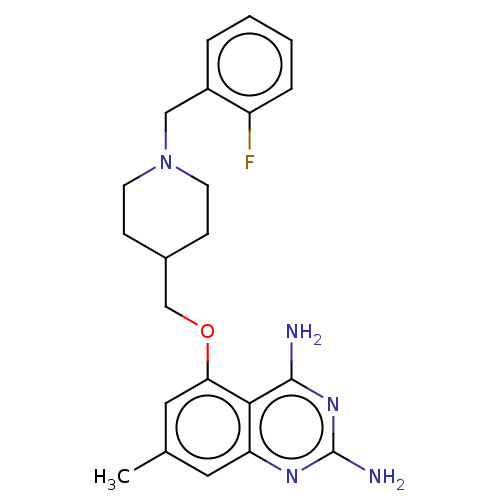
(CHEMBL4100442)Show SMILES Cc1cc(OCC2CCN(Cc3ccccc3F)CC2)c2c(N)nc(N)nc2c1 Show InChI InChI=1S/C22H26FN5O/c1-14-10-18-20(21(24)27-22(25)26-18)19(11-14)29-13-15-6-8-28(9-7-15)12-16-4-2-3-5-17(16)23/h2-5,10-11,15H,6-9,12-13H2,1H3,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237214
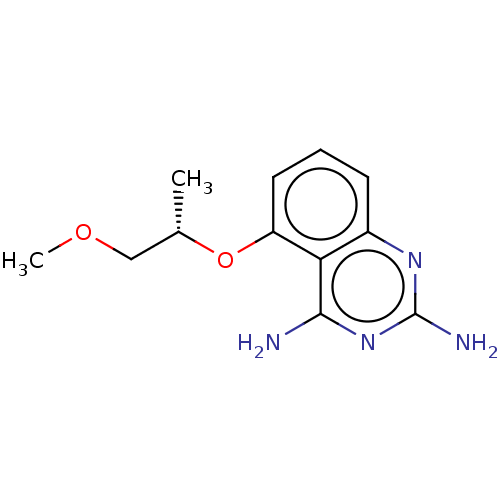
(CHEMBL4084161)Show InChI InChI=1S/C12H16N4O2/c1-7(6-17-2)18-9-5-3-4-8-10(9)11(13)16-12(14)15-8/h3-5,7H,6H2,1-2H3,(H4,13,14,15,16)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-HT3 receptor in rat was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237208
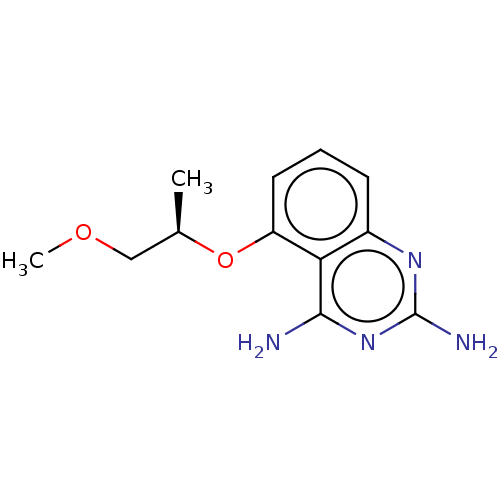
(CHEMBL4090641)Show InChI InChI=1S/C12H16N4O2/c1-7(6-17-2)18-9-5-3-4-8-10(9)11(13)16-12(14)15-8/h3-5,7H,6H2,1-2H3,(H4,13,14,15,16)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36531

(D158963)Show InChI InChI=1S/C16H20F3N5O/c17-16(18,19)9-24-6-4-10(5-7-24)8-25-12-3-1-2-11-13(12)14(20)23-15(21)22-11/h1-3,10H,4-9H2,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.4 | n/a | 223 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | CHEMBL4085817
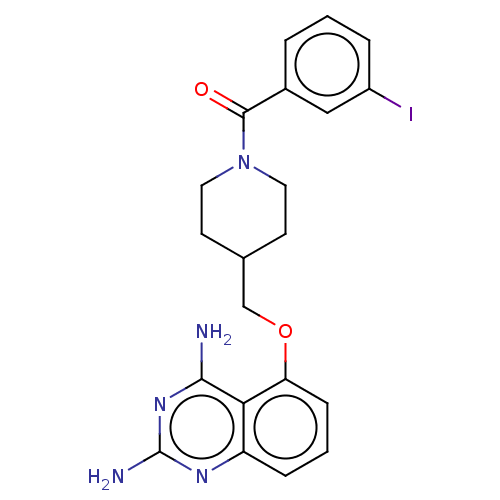
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36530

(D157493)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4cccc(Cl)c4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-4-1-3-14(19(15)23)11-28-9-7-13(8-10-28)12-29-17-6-2-5-16-18(17)20(24)27-21(25)26-16/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 43.9 | n/a | 67 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36532

(D158885)Show InChI InChI=1S/C20H22ClN5O/c21-14-4-1-2-6-16(14)26-10-8-13(9-11-26)12-27-17-7-3-5-15-18(17)19(22)25-20(23)24-15/h1-7,13H,8-12H2,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | 795 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM14323
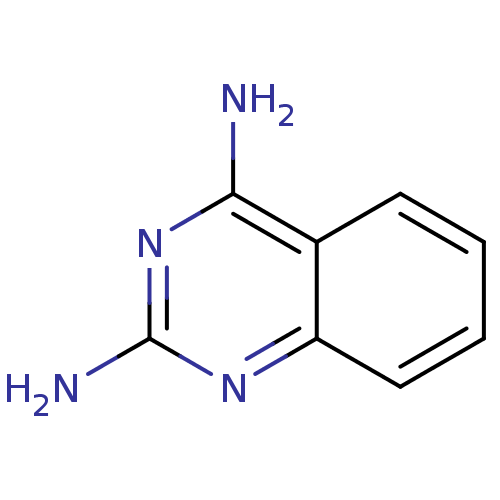
(Fragment 20 | quinazoline-2,4-diamine)Show InChI InChI=1S/C8H8N4/c9-7-5-3-1-2-4-6(5)11-8(10)12-7/h1-4H,(H4,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237219
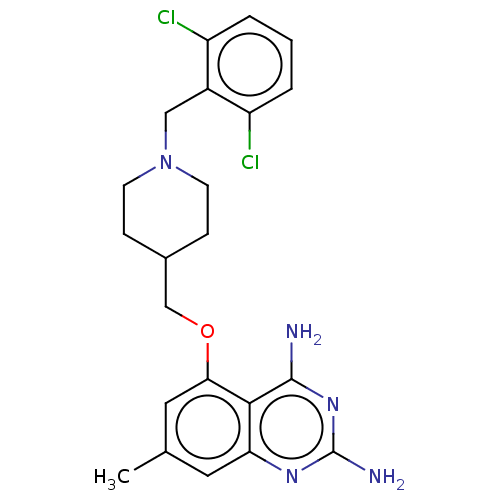
(CHEMBL4093512)Show SMILES Cc1cc(OCC2CCN(Cc3c(Cl)cccc3Cl)CC2)c2c(N)nc(N)nc2c1 Show InChI InChI=1S/C22H25Cl2N5O/c1-13-9-18-20(21(25)28-22(26)27-18)19(10-13)30-12-14-5-7-29(8-6-14)11-15-16(23)3-2-4-17(15)24/h2-4,9-10,14H,5-8,11-12H2,1H3,(H4,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36529

(D156676)Show SMILES CC(=O)Nc1nc(CN2CCC(COc3cccc4nc(N)nc(N)c34)CC2)cs1 Show InChI InChI=1S/C20H25N7O2S/c1-12(28)23-20-24-14(11-30-20)9-27-7-5-13(6-8-27)10-29-16-4-2-3-15-17(16)18(21)26-19(22)25-15/h2-4,11,13H,5-10H2,1H3,(H,23,24,28)(H4,21,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 339 | n/a | 2.54E+3 | n/a | n/a | n/a | n/a |
deCODE chemistry, Inc.
| Assay Description
In vitro biochemical assay using scavenger decapping enzyme (DcpS) as a target and cell based assay using SMN2 b-lactamase NSC-34 cell reporter gene. |
ACS Chem Biol 3: 711-22 (2008)
Article DOI: 10.1021/cb800120t
BindingDB Entry DOI: 10.7270/Q2Z036HN |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50316303

(((2R,3S,4R,5R)-5-(2-amino-7-methyl-6-oxo-1H-purin-...)Show SMILES C[n+]1cn([C@@H]2O[C@H](COP([O-])(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c2nc(N)[nH]c(=O)c12 |r| Show InChI InChI=1S/C11H17N5O11P2/c1-15-3-16(8-5(15)9(19)14-11(12)13-8)10-7(18)6(17)4(26-10)2-25-29(23,24)27-28(20,21)22/h3-4,6-7,10,17-18H,2H2,1H3,(H5-,12,13,14,19,20,21,22,23,24)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of human DCPS using m7GMPF as substrate after 30 mins by nucleoside 5'-fluorophosphate probe based HTS assay |
Bioorg Med Chem 26: 191-199 (2018)
Article DOI: 10.1016/j.bmc.2017.11.032
BindingDB Entry DOI: 10.7270/Q2DZ0BWS |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237213
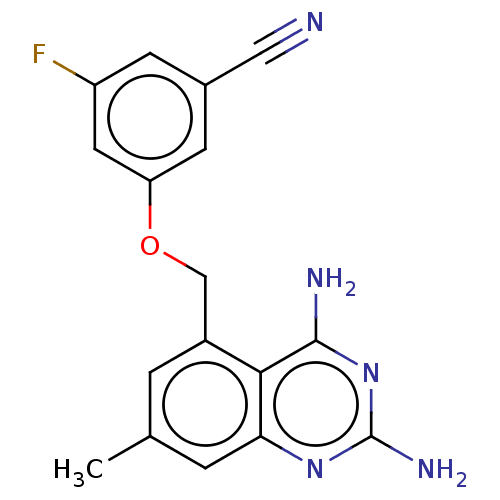
(CHEMBL4102384)Show InChI InChI=1S/C17H14FN5O/c1-9-2-11(15-14(3-9)22-17(21)23-16(15)20)8-24-13-5-10(7-19)4-12(18)6-13/h2-6H,8H2,1H3,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237215
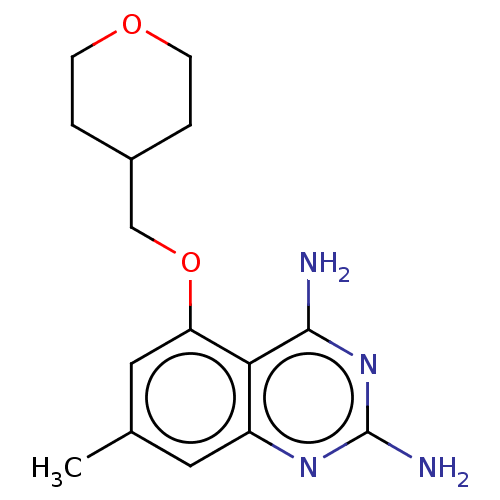
(CHEMBL4075512)Show InChI InChI=1S/C15H20N4O2/c1-9-6-11-13(14(16)19-15(17)18-11)12(7-9)21-8-10-2-4-20-5-3-10/h6-7,10H,2-5,8H2,1H3,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50316304
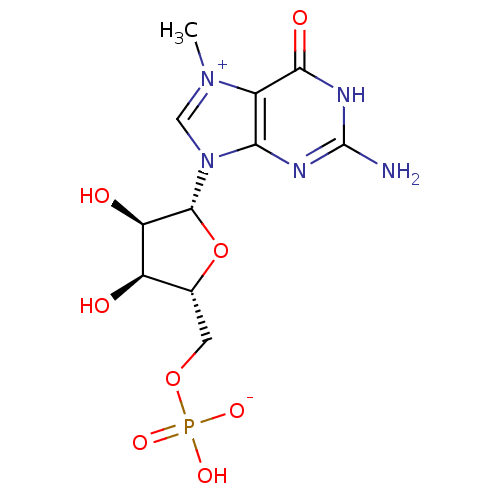
(((2R,3S,4R,5R)-5-(2-amino-7-methyl-6-oxo-1H-purin-...)Show SMILES C[n+]1cn([C@@H]2O[C@H](COP(O)([O-])=O)[C@@H](O)[C@H]2O)c2nc(N)[nH]c(=O)c12 |r| Show InChI InChI=1S/C11H16N5O8P/c1-15-3-16(8-5(15)9(19)14-11(12)13-8)10-7(18)6(17)4(24-10)2-23-25(20,21)22/h3-4,6-7,10,17-18H,2H2,1H3,(H4-,12,13,14,19,20,21,22)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of human DCPS using m7GMPF as substrate after 30 mins by nucleoside 5'-fluorophosphate probe based HTS assay |
Bioorg Med Chem 26: 191-199 (2018)
Article DOI: 10.1016/j.bmc.2017.11.032
BindingDB Entry DOI: 10.7270/Q2DZ0BWS |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50452630

(CHEMBL4203412)Show SMILES C[n+]1cn([C@@H]2O[C@H](Cn3cc(nn3)P(O)([O-])=O)[C@@H](O)[C@H]2O)c2nc(N)[nH]c(=O)c12 |r| Show InChI InChI=1S/C13H17N8O7P/c1-19-4-21(10-7(19)11(24)16-13(14)15-10)12-9(23)8(22)5(28-12)2-20-3-6(17-18-20)29(25,26)27/h3-5,8-9,12,22-23H,2H2,1H3,(H4-,14,15,16,24,25,26,27)/t5-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of human DCPS using m7GMPF as substrate after 30 mins by nucleoside 5'-fluorophosphate probe based HTS assay |
Bioorg Med Chem 26: 191-199 (2018)
Article DOI: 10.1016/j.bmc.2017.11.032
BindingDB Entry DOI: 10.7270/Q2DZ0BWS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data