 Found 332 hits of ic50 for UniProtKB: O94806
Found 332 hits of ic50 for UniProtKB: O94806 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition Protein kinase C (PKC) |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519062

(CHEMBL4442196)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(C)=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:41:57.58:38,THB:62:43:57.58:38,45:43:57.58:38,56:57:43.41.44:38,32:37:45.57.56:41| Show InChI InChI=1S/C46H56O13/c1-25(2)42-22-31(23-53-39(49)29-17-11-8-12-18-29)45-34-37(42)57-46(58-42,59-45)32(54-28(5)48)21-15-7-6-10-16-26(3)33-27(4)36(55-40(50)30-19-13-9-14-20-30)44(52,35(33)45)41(51)43(24-47)38(34)56-43/h8-9,11-14,17-20,26-27,31-38,41,47,51-52H,1,6-7,10,15-16,21-24H2,2-5H3/t26-,27+,31+,32-,33+,34-,35-,36+,37-,38+,41-,42-,43+,44-,45-,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519087
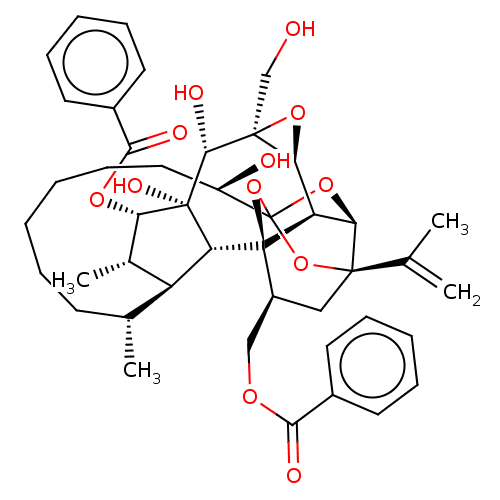
(CHEMBL3741746)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:35:54.55,THB:55:34:38:54.53.42,32:34:38:54.53.42,53:54:40.38.41:35,42:40:35:54.55,56:54:40.38.41:35| Show InChI InChI=1S/C44H54O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,39,45-46,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519068
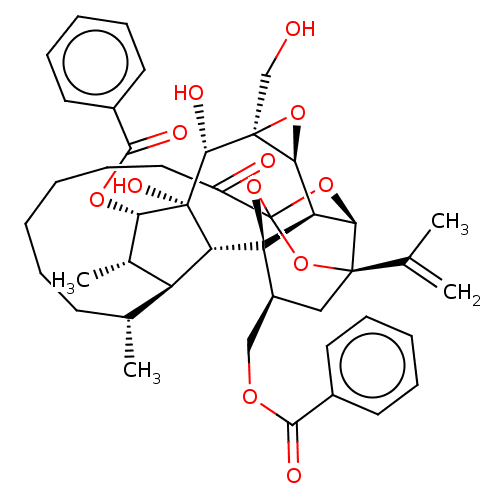
(CHEMBL4575056)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCCC(=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29,31-36,39,45,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519060
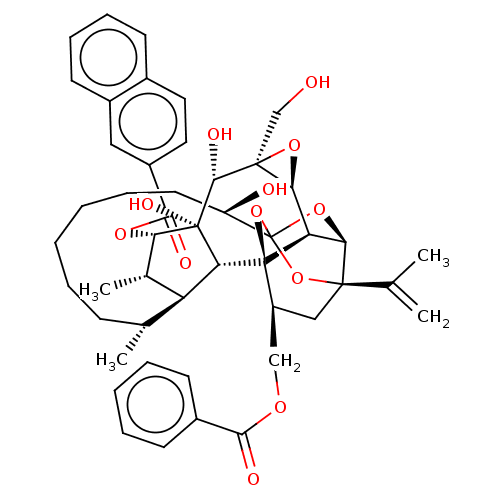
(CHEMBL4528495)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccc4ccccc4c3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:42:58.59:39,THB:63:44:58.59:39,36:38:46.58.57:42,46:44:58.59:39,57:58:44.42.45:39| Show InChI InChI=1S/C48H56O12/c1-26(2)44-23-33(24-55-41(51)30-16-9-7-10-17-30)47-36-39(44)58-48(59-44,60-47)34(50)19-11-6-5-8-14-27(3)35-28(4)38(46(54,37(35)47)43(53)45(25-49)40(36)57-45)56-42(52)32-21-20-29-15-12-13-18-31(29)22-32/h7,9-10,12-13,15-18,20-22,27-28,33-40,43,49-50,53-54H,1,5-6,8,11,14,19,23-25H2,2-4H3/t27-,28+,33+,34-,35+,36-,37-,38+,39-,40+,43-,44-,45+,46-,47-,48?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519083

(CHEMBL4538431)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccc(OC)cc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:40:56.57:37,THB:61:42:56.57:37,44:42:56.57:37,55:56:42.40.43:37,34:36:44.56.55:40| Show InChI InChI=1S/C45H56O13/c1-24(2)41-21-29(22-53-38(48)27-14-10-8-11-15-27)44-33-36(41)56-45(57-41,58-44)31(47)16-12-7-6-9-13-25(3)32-26(4)35(54-39(49)28-17-19-30(52-5)20-18-28)43(51,34(32)44)40(50)42(23-46)37(33)55-42/h8,10-11,14-15,17-20,25-26,29,31-37,40,46-47,50-51H,1,6-7,9,12-13,16,21-23H2,2-5H3/t25-,26+,29+,31-,32+,33-,34-,35+,36-,37+,40-,41-,42+,43-,44-,45?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519084
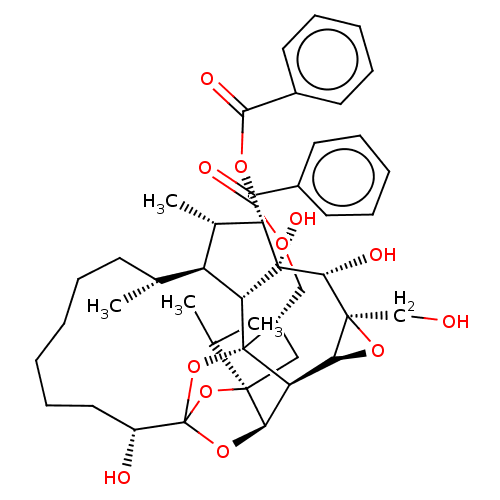
(CHEMBL4443190)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H56O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,24-26,29-36,39,45-46,49-50H,5-6,9,14-15,20-23H2,1-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519069
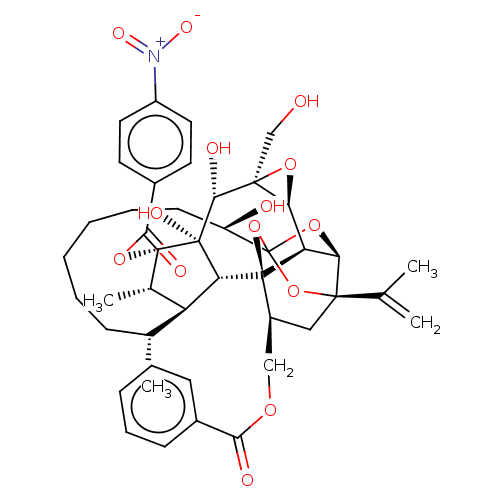
(CHEMBL4435580)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccc(cc3)[N+]([O-])=O)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:41:57.58:38,THB:62:43:57.58:38,45:43:57.58:38,56:57:43.41.44:38,35:37:45.57.56:41| Show InChI InChI=1S/C44H53NO14/c1-23(2)40-20-28(21-54-37(48)26-13-9-7-10-14-26)43-32-35(40)57-44(58-40,59-43)30(47)15-11-6-5-8-12-24(3)31-25(4)34(55-38(49)27-16-18-29(19-17-27)45(52)53)42(51,33(31)43)39(50)41(22-46)36(32)56-41/h7,9-10,13-14,16-19,24-25,28,30-36,39,46-47,50-51H,1,5-6,8,11-12,15,20-22H2,2-4H3/t24-,25+,28+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519063
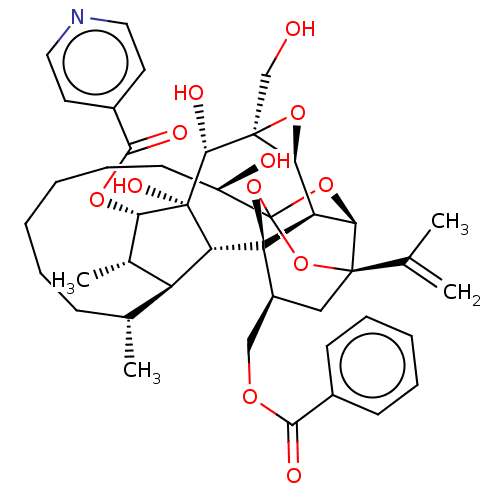
(CHEMBL4587471)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccncc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C43H53NO12/c1-23(2)39-20-28(21-51-36(47)26-13-9-7-10-14-26)42-31-34(39)54-43(55-39,56-42)29(46)15-11-6-5-8-12-24(3)30-25(4)33(52-37(48)27-16-18-44-19-17-27)41(50,32(30)42)38(49)40(22-45)35(31)53-40/h7,9-10,13-14,16-19,24-25,28-35,38,45-46,49-50H,1,5-6,8,11-12,15,20-22H2,2-4H3/t24-,25+,28+,29-,30+,31-,32-,33+,34-,35+,38-,39-,40+,41-,42-,43?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27946V3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519089
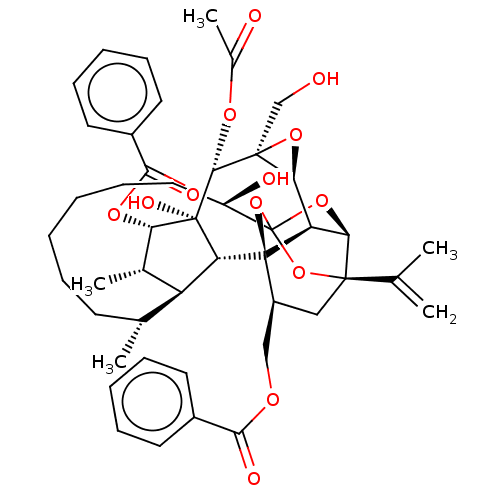
(CHEMBL4560772)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](OC(C)=O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:41:57.58:38,THB:62:43:57.58:38,45:43:57.58:38,56:57:43.41.44:38,35:37:45.57.56:41| Show InChI InChI=1S/C46H56O13/c1-25(2)42-22-31(23-53-39(50)29-17-11-8-12-18-29)45-34-37(42)57-46(58-42,59-45)32(49)21-15-7-6-10-16-26(3)33-27(4)36(55-40(51)30-19-13-9-14-20-30)44(52,35(33)45)41(54-28(5)48)43(24-47)38(34)56-43/h8-9,11-14,17-20,26-27,31-38,41,47,49,52H,1,6-7,10,15-16,21-24H2,2-5H3/t26-,27+,31+,32-,33+,34-,35-,36+,37-,38+,41-,42-,43+,44-,45-,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519064
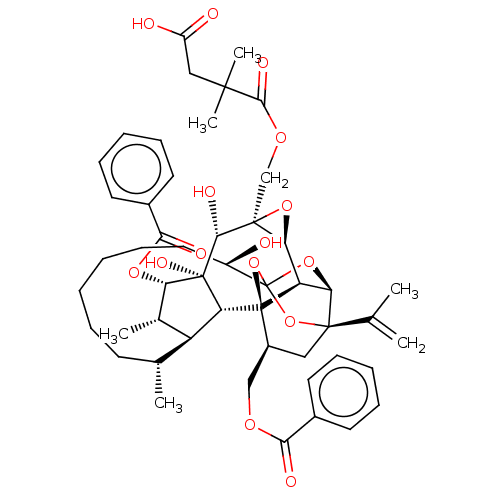
(CHEMBL4586553)Show SMILES [H][C@@]12O[C@]1(COC(=O)C(C)(C)CC(O)=O)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:47:63.64:44,THB:68:49:63.64:44,51:49:63.64:44,62:63:49.47.50:44,41:43:51.63.62:47| Show InChI InChI=1S/C50H62O15/c1-27(2)46-23-32(25-59-41(54)30-18-12-9-13-19-30)49-36-39(46)63-50(64-46,65-49)33(51)22-16-8-7-11-17-28(3)35-29(4)38(61-42(55)31-20-14-10-15-21-31)48(58,37(35)49)43(56)47(40(36)62-47)26-60-44(57)45(5,6)24-34(52)53/h9-10,12-15,18-21,28-29,32-33,35-40,43,51,56,58H,1,7-8,11,16-17,22-26H2,2-6H3,(H,52,53)/t28-,29+,32+,33-,35+,36-,37-,38+,39-,40+,43-,46-,47+,48-,49-,50?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519088
(CHEMBL4475196)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)c4ccc(cc4)[N+]([O-])=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:49:65.66:46,THB:70:51:65.66:46,53:51:65.66:46,64:65:51.49.52:46,32:45:53.65.64:49| Show InChI InChI=1S/C51H57NO15/c1-28(2)47-25-34(26-61-43(54)31-16-10-7-11-17-31)50-38-41(47)65-51(66-47,67-50)36(62-44(55)33-21-23-35(24-22-33)52(59)60)20-14-6-5-9-15-29(3)37-30(4)40(63-45(56)32-18-12-8-13-19-32)49(58,39(37)50)46(57)48(27-53)42(38)64-48/h7-8,10-13,16-19,21-24,29-30,34,36-42,46,53,57-58H,1,5-6,9,14-15,20,25-27H2,2-4H3/t29-,30+,34+,36-,37+,38-,39-,40+,41-,42+,46-,47-,48+,49-,50-,51?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519086
(CHEMBL4460901)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](O)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](CO)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:30:38.39:27,THB:34:32:38.39:27,37:38:32.30.33:27,43:32:38.39:27,24:26:34.38.37:30| Show InChI InChI=1S/C30H46O10/c1-14(2)26-11-17(12-31)29-20-23(26)38-30(39-26,40-29)18(33)10-8-6-5-7-9-15(3)19-16(4)22(34)28(36,21(19)29)25(35)27(13-32)24(20)37-27/h15-25,31-36H,1,5-13H2,2-4H3/t15-,16+,17+,18-,19+,20-,21-,22+,23-,24+,25-,26-,27+,28-,29-,30?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519085

(CHEMBL4444766)Show SMILES [H][C@@]12O[C@]1(CO)C(=O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,45-46,50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519082

(CHEMBL4527508)Show SMILES [H][C@@]12O[C@]1(COC(=O)c1ccccc1)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:46:62.63:43,THB:67:48:62.63:43,50:48:62.63:43,61:62:48.46.49:43,40:42:50.62.61:46| Show InChI InChI=1S/C51H58O13/c1-29(2)47-26-35(27-58-43(53)32-19-11-7-12-20-32)50-38-41(47)62-51(63-47,64-50)36(52)25-17-6-5-10-18-30(3)37-31(4)40(60-45(55)34-23-15-9-16-24-34)49(57,39(37)50)46(56)48(42(38)61-48)28-59-44(54)33-21-13-8-14-22-33/h7-9,11-16,19-24,30-31,35-42,46,52,56-57H,1,5-6,10,17-18,25-28H2,2-4H3/t30-,31+,35+,36-,37+,38-,39-,40+,41-,42+,46-,47-,48+,49-,50-,51?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519081

(CHEMBL4537465)Show SMILES [H][C@@]12O[C@]1(COC(C)=O)[C@@H](OC(C)=O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(C)=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:47:63.64:44,THB:68:49:63.64:44,51:49:63.64:44,62:63:49.47.50:44,38:43:51.63.62:47| Show InChI InChI=1S/C50H60O15/c1-27(2)46-24-35(25-57-43(54)33-19-13-10-14-20-33)49-38-41(46)63-50(64-46,65-49)36(59-31(6)52)23-17-9-8-12-18-28(3)37-29(4)40(61-44(55)34-21-15-11-16-22-34)48(56,39(37)49)45(60-32(7)53)47(42(38)62-47)26-58-30(5)51/h10-11,13-16,19-22,28-29,35-42,45,56H,1,8-9,12,17-18,23-26H2,2-7H3/t28-,29+,35+,36-,37+,38-,39-,40+,41-,42+,45-,46-,47+,48-,49-,50?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519080
(CHEMBL4562111)Show SMILES [H][C@@]12O[C@]1(COC(=O)c1ccccc1)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)c4ccccc4)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:54:70.71:51,THB:75:56:70.71:51,58:56:70.71:51,69:70:56.54.57:51,40:50:58.70.69:54| Show InChI InChI=1S/C58H62O14/c1-34(2)54-31-41(32-65-49(59)37-22-12-7-13-23-37)57-44-47(54)70-58(71-54,72-57)42(67-51(61)39-26-16-9-17-27-39)30-20-6-5-11-21-35(3)43-36(4)46(68-52(62)40-28-18-10-19-29-40)56(64,45(43)57)53(63)55(48(44)69-55)33-66-50(60)38-24-14-8-15-25-38/h7-10,12-19,22-29,35-36,41-48,53,63-64H,1,5-6,11,20-21,30-33H2,2-4H3/t35-,36+,41+,42-,43+,44-,45-,46+,47-,48+,53-,54-,55+,56-,57-,58?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519079

(CHEMBL4451784)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)c4ccc(OC)cc4)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:48:64.65:45,THB:69:50:64.65:45,52:50:64.65:45,63:64:50.48.51:45,32:44:52.64.63:48| Show InChI InChI=1S/C52H60O14/c1-29(2)48-26-35(27-60-44(54)32-17-11-8-12-18-32)51-39-42(48)64-52(65-48,66-51)37(61-45(55)34-22-24-36(59-5)25-23-34)21-15-7-6-10-16-30(3)38-31(4)41(62-46(56)33-19-13-9-14-20-33)50(58,40(38)51)47(57)49(28-53)43(39)63-49/h8-9,11-14,17-20,22-25,30-31,35,37-43,47,53,57-58H,1,6-7,10,15-16,21,26-28H2,2-5H3/t30-,31+,35+,37-,38+,39-,40-,41+,42-,43+,47-,48-,49+,50-,51-,52?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519078
(CHEMBL4587134)Show SMILES [H][C@@]12O[C@]1(COC(=O)CC(C)(C)C(O)=O)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:47:63.64:44,THB:68:49:63.64:44,51:49:63.64:44,62:63:49.47.50:44,41:43:51.63.62:47| Show InChI InChI=1S/C50H62O15/c1-27(2)46-23-32(25-59-41(53)30-18-12-9-13-19-30)49-36-39(46)63-50(64-46,65-49)33(51)22-16-8-7-11-17-28(3)35-29(4)38(61-42(54)31-20-14-10-15-21-31)48(58,37(35)49)43(55)47(40(36)62-47)26-60-34(52)24-45(5,6)44(56)57/h9-10,12-15,18-21,28-29,32-33,35-40,43,51,55,58H,1,7-8,11,16-17,22-26H2,2-6H3,(H,56,57)/t28-,29+,32+,33-,35+,36-,37-,38+,39-,40+,43-,46-,47+,48-,49-,50?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519077
(CHEMBL4470135)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](OC(=O)c1ccccc1)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:46:62.63:43,THB:67:48:62.63:43,50:48:62.63:43,61:62:48.46.49:43,40:42:50.62.61:46| Show InChI InChI=1S/C51H58O13/c1-29(2)47-26-35(27-58-43(54)32-19-11-7-12-20-32)50-38-41(47)62-51(63-47,64-50)36(53)25-17-6-5-10-18-30(3)37-31(4)40(59-44(55)33-21-13-8-14-22-33)49(57,39(37)50)46(48(28-52)42(38)61-48)60-45(56)34-23-15-9-16-24-34/h7-9,11-16,19-24,30-31,35-42,46,52-53,57H,1,5-6,10,17-18,25-28H2,2-4H3/t30-,31+,35+,36-,37+,38-,39-,40+,41-,42+,46-,47-,48+,49-,50-,51?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519076
(CHEMBL4570883)Show SMILES [H][C@@]12O[C@]1(COC(=O)[C@@]13CC[C@@](C)(C(=O)O1)C3(C)C)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:14:13:16:10.9,1:51:67.68:48,THB:55:53:67.68:48,66:67:53.51.54:48,45:47:55.67.66:51,72:53:67.68:48| Show InChI InChI=1S/C54H66O15/c1-29(2)49-26-34(27-62-42(56)32-19-13-10-14-20-32)53-37-40(49)66-54(68-49,69-53)35(55)23-17-9-8-12-18-30(3)36-31(4)39(64-43(57)33-21-15-11-16-22-33)52(61,38(36)53)44(58)50(41(37)65-50)28-63-46(60)51-25-24-48(7,45(59)67-51)47(51,5)6/h10-11,13-16,19-22,30-31,34-41,44,55,58,61H,1,8-9,12,17-18,23-28H2,2-7H3/t30-,31+,34+,35-,36+,37-,38-,39+,40-,41+,44-,48+,49-,50+,51-,52-,53-,54?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519075
(CHEMBL4558004)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](O)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:30:46.47:27,THB:51:32:46.47:27,24:26:34.46.45:30,34:32:46.47:27,45:46:32.30.33:27| Show InChI InChI=1S/C37H50O11/c1-19(2)33-16-23(17-44-31(41)22-13-9-7-10-14-22)36-26-29(33)46-37(47-33,48-36)24(39)15-11-6-5-8-12-20(3)25-21(4)28(40)35(43,27(25)36)32(42)34(18-38)30(26)45-34/h7,9-10,13-14,20-21,23-30,32,38-40,42-43H,1,5-6,8,11-12,15-18H2,2-4H3/t20-,21+,23+,24-,25+,26-,27-,28+,29-,30+,32-,33-,34+,35-,36-,37?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519074

(CHEMBL4442856)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)\C=C\c4ccccc4)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:48:64.65:45,THB:69:50:64.65:45,52:50:64.65:45,63:64:50.48.51:45,32:44:52.64.63:48| Show InChI InChI=1S/C53H60O13/c1-31(2)49-28-37(29-60-46(56)35-21-13-8-14-22-35)52-41-44(49)64-53(65-49,66-52)38(61-39(55)27-26-34-19-11-7-12-20-34)25-17-6-5-10-18-32(3)40-33(4)43(62-47(57)36-23-15-9-16-24-36)51(59,42(40)52)48(58)50(30-54)45(41)63-50/h7-9,11-16,19-24,26-27,32-33,37-38,40-45,48,54,58-59H,1,5-6,10,17-18,25,28-30H2,2-4H3/b27-26+/t32-,33+,37+,38-,40+,41-,42-,43+,44-,45+,48-,49-,50+,51-,52-,53?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519073

(CHEMBL4536366)Show SMILES [H][C@@]12O[C@]1(COC(=O)C1(C)COC(C)(C)OC1)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:49:65.66:46,THB:70:51:65.66:46,53:51:65.66:46,64:65:51.49.52:46,43:45:53.65.64:49| Show InChI InChI=1S/C52H66O15/c1-29(2)48-24-34(25-59-42(54)32-19-13-10-14-20-32)51-37-40(48)65-52(66-48,67-51)35(53)23-17-9-8-12-18-30(3)36-31(4)39(63-43(55)33-21-15-11-16-22-33)50(58,38(36)51)44(56)49(41(37)64-49)28-60-45(57)47(7)26-61-46(5,6)62-27-47/h10-11,13-16,19-22,30-31,34-41,44,53,56,58H,1,8-9,12,17-18,23-28H2,2-7H3/t30-,31+,34+,35-,36+,37-,38-,39+,40-,41+,44-,48-,49+,50-,51-,52?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519072
(CHEMBL4580556)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)c4ccc5ccccc5c4)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:50:66.67:47,THB:71:52:66.67:47,54:52:66.67:47,65:66:52.50.53:47,32:46:54.66.65:50| Show InChI InChI=1S/C55H60O13/c1-31(2)51-28-39(29-62-47(57)35-19-10-7-11-20-35)54-42-45(51)66-55(67-51,68-54)40(63-49(59)38-26-25-34-18-15-16-23-37(34)27-38)24-14-6-5-9-17-32(3)41-33(4)44(64-48(58)36-21-12-8-13-22-36)53(61,43(41)54)50(60)52(30-56)46(42)65-52/h7-8,10-13,15-16,18-23,25-27,32-33,39-46,50,56,60-61H,1,5-6,9,14,17,24,28-30H2,2-4H3/t32-,33+,39+,40-,41+,42-,43-,44+,45-,46+,50-,51-,52+,53-,54-,55?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519071
(CHEMBL4585270)Show SMILES [H][C@@]12O[C@]1(COC(=O)\C=C\c1ccc(OC)cc1)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:50:66.67:47,THB:71:52:66.67:47,54:52:66.67:47,65:66:52.50.53:47,44:46:54.66.65:50| Show InChI InChI=1S/C54H62O14/c1-31(2)50-28-37(29-62-47(57)35-17-11-8-12-18-35)53-42-45(50)66-54(67-50,68-53)39(55)21-15-7-6-10-16-32(3)41-33(4)44(64-48(58)36-19-13-9-14-20-36)52(60,43(41)53)49(59)51(46(42)65-51)30-63-40(56)27-24-34-22-25-38(61-5)26-23-34/h8-9,11-14,17-20,22-27,32-33,37,39,41-46,49,55,59-60H,1,6-7,10,15-16,21,28-30H2,2-5H3/b27-24+/t32-,33+,37+,39-,41+,42-,43-,44+,45-,46+,49-,50-,51+,52-,53-,54?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519070

(CHEMBL4580586)Show SMILES [H][C@@]12O[C@]1(COC(C)=O)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:41:57.58:38,THB:62:43:57.58:38,45:43:57.58:38,56:57:43.41.44:38,35:37:45.57.56:41| Show InChI InChI=1S/C46H56O13/c1-25(2)42-22-31(23-53-39(49)29-17-11-8-12-18-29)45-34-37(42)57-46(58-42,59-45)32(48)21-15-7-6-10-16-26(3)33-27(4)36(55-40(50)30-19-13-9-14-20-30)44(52,35(33)45)41(51)43(38(34)56-43)24-54-28(5)47/h8-9,11-14,17-20,26-27,31-38,41,48,51-52H,1,6-7,10,15-16,21-24H2,2-5H3/t26-,27+,31+,32-,33+,34-,35-,36+,37-,38+,41-,42-,43+,44-,45-,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519067
(CHEMBL4443186)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)C(\C)=C\C)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:44:60.61:41,THB:65:46:60.61:41,48:46:60.61:41,59:60:46.44.47:41,32:40:48.60.59:44| Show InChI InChI=1S/C49H60O13/c1-7-28(4)41(51)57-34-23-17-9-8-12-18-29(5)35-30(6)38(58-43(53)32-21-15-11-16-22-32)47(55)37(35)48-33(25-56-42(52)31-19-13-10-14-20-31)24-45(27(2)3)39(60-49(34,61-45)62-48)36(48)40-46(26-50,59-40)44(47)54/h7,10-11,13-16,19-22,29-30,33-40,44,50,54-55H,2,8-9,12,17-18,23-26H2,1,3-6H3/b28-7+/t29-,30+,33+,34-,35+,36-,37-,38+,39-,40+,44-,45-,46+,47-,48-,49?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519066
(CHEMBL4460926)Show SMILES [H][C@@]12O[C@]1(COC(=O)CCC(O)=O)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:45:61.62:42,THB:66:47:61.62:42,49:47:61.62:42,60:61:47.45.48:42,39:41:49.61.60:45| Show InChI InChI=1S/C48H58O15/c1-26(2)44-23-31(24-57-41(53)29-16-10-7-11-17-29)47-36-39(44)61-48(62-44,63-47)32(49)20-14-6-5-9-15-27(3)35-28(4)38(59-42(54)30-18-12-8-13-19-30)46(56,37(35)47)43(55)45(40(36)60-45)25-58-34(52)22-21-33(50)51/h7-8,10-13,16-19,27-28,31-32,35-40,43,49,55-56H,1,5-6,9,14-15,20-25H2,2-4H3,(H,50,51)/t27-,28+,31+,32-,35+,36-,37-,38+,39-,40+,43-,44-,45+,46-,47-,48?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519065

(CHEMBL4578026)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(=O)c4ccccc4)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:46:62.63:43,THB:67:48:62.63:43,50:48:62.63:43,61:62:48.46.49:43,32:42:50.62.61:46| Show InChI InChI=1S/C51H58O13/c1-29(2)47-26-35(27-58-43(53)32-19-11-7-12-20-32)50-38-41(47)62-51(63-47,64-50)36(59-44(54)33-21-13-8-14-22-33)25-17-6-5-10-18-30(3)37-31(4)40(60-45(55)34-23-15-9-16-24-34)49(57,39(37)50)46(56)48(28-52)42(38)61-48/h7-9,11-16,19-24,30-31,35-42,46,52,56-57H,1,5-6,10,17-18,25-28H2,2-4H3/t30-,31+,35+,36-,37+,38-,39-,40+,41-,42+,46-,47-,48+,49-,50-,51?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519061

(CHEMBL4533733)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)\C=C\c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:40:56.57:37,THB:61:42:56.57:37,34:36:44.56.55:40,44:42:56.57:37,55:56:42.40.43:37| Show InChI InChI=1S/C46H56O12/c1-26(2)42-23-31(24-53-40(50)30-18-12-8-13-19-30)45-35-38(42)56-46(57-42,58-45)32(48)20-14-6-5-9-15-27(3)34-28(4)37(54-33(49)22-21-29-16-10-7-11-17-29)44(52,36(34)45)41(51)43(25-47)39(35)55-43/h7-8,10-13,16-19,21-22,27-28,31-32,34-39,41,47-48,51-52H,1,5-6,9,14-15,20,23-25H2,2-4H3/b22-21+/t27-,28+,31+,32-,34+,35-,36-,37+,38-,39+,41-,42-,43+,44-,45-,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human PKCnu using KKLNRTLSVA as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PKCnu using KKLNRTLSVA as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01031
BindingDB Entry DOI: 10.7270/Q2FF3XF8 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50213014
(CHEMBL83790)Show SMILES CN[C@H]1CC2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5cc(O)ccc5n2c4c13 Show InChI InChI=1S/C28H26N4O4/c1-28-26(35-3)17(29-2)11-20(36-28)31-18-9-8-13(33)10-15(18)22-23-16(12-30-27(23)34)21-14-6-4-5-7-19(14)32(28)25(21)24(22)31/h4-10,17,20,26,29,33H,11-12H2,1-3H3,(H,30,34)/t17-,20?,26-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27946V3 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2HX1FVJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of Protein Kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2HM5BN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM50565693
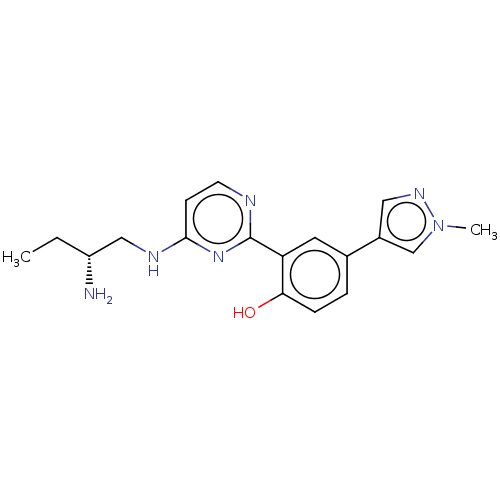
(CHEMBL4515390)Show SMILES CC[C@@H](N)CNc1ccnc(n1)-c1cc(ccc1O)-c1cnn(C)c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant FLAG-tagged PKD3 (unknown origin) expressed in HEK293T cells using Syntide-2 as substrate measured after 15 mins by ADP-Glo... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112638
BindingDB Entry DOI: 10.7270/Q21V5JQG |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin E-cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C (PKC) isozyme |
Citation and Details
Article DOI: 10.1016/0960-894X(94)80024-3
BindingDB Entry DOI: 10.7270/Q2D50QPQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50213033
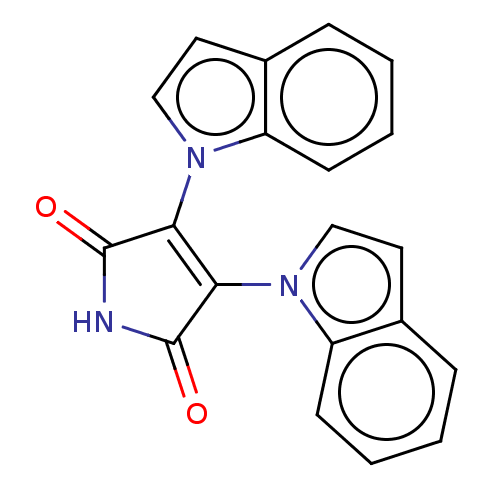
(CHEMBL329274)Show SMILES O=C1NC(=O)C(=C1n1ccc2ccccc12)n1ccc2ccccc12 |c:5| Show InChI InChI=1S/C20H13N3O2/c24-19-17(22-11-9-13-5-1-3-7-15(13)22)18(20(25)21-19)23-12-10-14-6-2-4-8-16(14)23/h1-12H,(H,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C (PKC) using histone III S protein |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZS2ZQ0 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50528409

(CHEMBL4441825)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@@H]1CN[C@H](CCO)C[C@H]1c1ccc(F)c(F)c1 |r,wU:16.17,19.21,wD:24.27,(5.73,-5.85,;5.41,-7.35,;4,-7.98,;4.16,-9.51,;5.66,-9.83,;6.29,-11.24,;6.44,-8.5,;7.97,-8.34,;9,-9.49,;10.41,-8.86,;10.25,-7.33,;8.75,-7.01,;8.12,-5.6,;11.75,-9.64,;11.74,-11.18,;13.08,-8.87,;14.41,-9.65,;14.41,-11.18,;15.74,-11.96,;17.08,-11.19,;18.41,-11.96,;19.74,-11.2,;21.07,-11.97,;17.08,-9.64,;15.75,-8.88,;15.75,-7.35,;14.42,-6.58,;14.42,-5.03,;15.75,-4.26,;15.74,-2.72,;17.08,-5.03,;18.41,-4.25,;17.09,-6.58,)| Show InChI InChI=1S/C22H22Cl2F2N4O3/c1-30-20(15(23)9-28-30)14-8-19(33-21(14)24)22(32)29-18-10-27-12(4-5-31)7-13(18)11-2-3-16(25)17(26)6-11/h2-3,6,8-9,12-13,18,27,31H,4-5,7,10H2,1H3,(H,29,32)/t12-,13+,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PKC (unknown origin) |
J Med Chem 62: 7264-7288 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00891
BindingDB Entry DOI: 10.7270/Q2XW4P7W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM50337694

((R)-N-(1-cyanoethyl)-3-(5-(4-((tetrahydro-2H-pyran...)Show SMILES C[C@@H](NC(=O)c1cccc(c1)-c1cc(on1)-c1ccc(CNC2CCOCC2)cc1)C#N |r| Show InChI InChI=1S/C25H26N4O3/c1-17(15-26)28-25(30)21-4-2-3-20(13-21)23-14-24(32-29-23)19-7-5-18(6-8-19)16-27-22-9-11-31-12-10-22/h2-8,13-14,17,22,27H,9-12,16H2,1H3,(H,28,30)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PKD3 |
Bioorg Med Chem Lett 21: 1447-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.014
BindingDB Entry DOI: 10.7270/Q2JM29WX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM50565690

(CHEMBL4787572)Show SMILES Nc1ncnc2n(CC3CCNCC3)nc(-c3c[nH]c4ccccc34)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant FLAG-tagged PKD3 (unknown origin) expressed in HEK293T cells using Syntide-2 as substrate measured after 15 mins by ADP-Glo... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112638
BindingDB Entry DOI: 10.7270/Q21V5JQG |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C(PKC) |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2542QS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27946V3 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50469707

(CHEMBL147986)Show SMILES [H][C@]12CCC[C@]([H])(N1)n1c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C25H18N4O2/c30-24-20-18-12-6-1-3-8-14(12)28-16-10-5-11-17(26-16)29-15-9-4-2-7-13(15)19(23(29)22(18)28)21(20)25(31)27-24/h1-4,6-9,16-17,26H,5,10-11H2,(H,27,30,31)/t16-,17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C (PKC) isozyme |
Citation and Details
Article DOI: 10.1016/0960-894X(94)80024-3
BindingDB Entry DOI: 10.7270/Q2D50QPQ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2HX1FVJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50212473
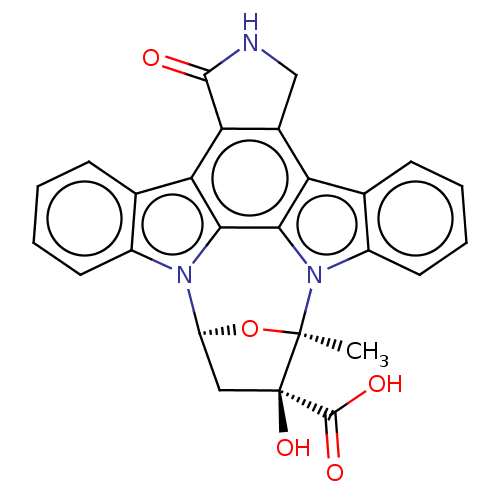
(CHEMBL140600)Show SMILES [H][C@@]12C[C@@](O)(C(O)=O)[C@@](C)(O1)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C26H19N3O5/c1-25-26(33,24(31)32)10-17(34-25)28-15-8-4-2-6-12(15)19-20-14(11-27-23(20)30)18-13-7-3-5-9-16(13)29(25)22(18)21(19)28/h2-9,17,33H,10-11H2,1H3,(H,27,30)(H,31,32)/t17-,25+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of protein kinase C |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2HX1FVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data