

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50585203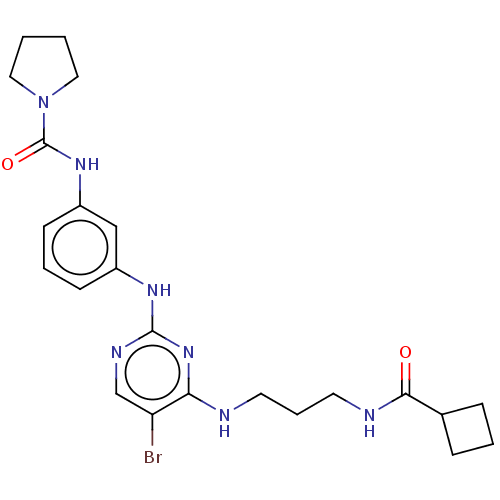 (CHEMBL5085430) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of tracer K9 from NLuc fused DRAK1 (unknown origin) expressed in HEK293 cells by NanoBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00440 BindingDB Entry DOI: 10.7270/Q27S7SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50274640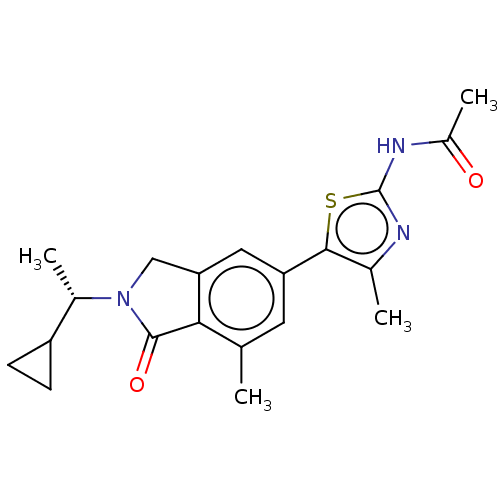 (CHEMBL4126445) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged DRAK1 expressed in baculovirus expression system | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441855 (US10647686, Example (3)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50166076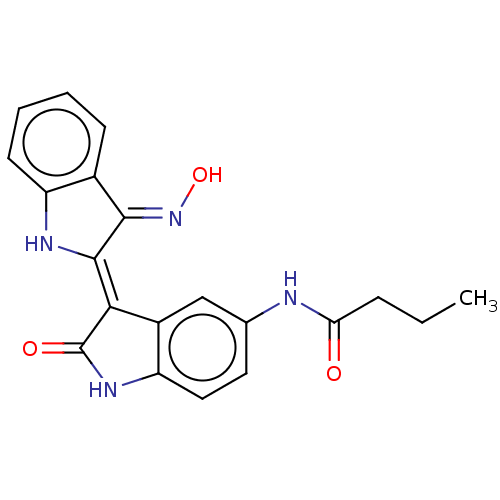 (CHEMBL3799585) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK1 (unknown origin) | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50154342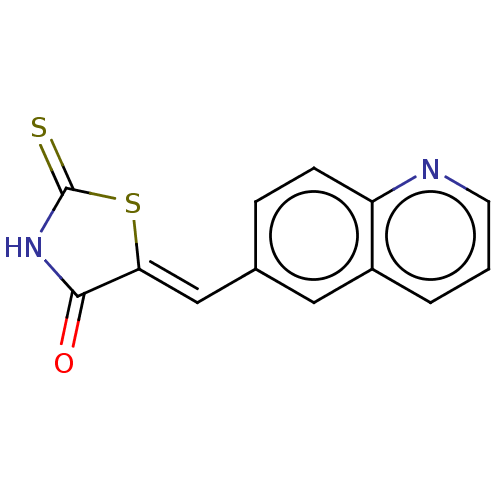 (CHEMBL3774448) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of STK17A (unknown origin) | Eur J Med Chem 112: 209-16 (2016) Article DOI: 10.1016/j.ejmech.2016.02.017 BindingDB Entry DOI: 10.7270/Q2GF0WC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441866 (US10647686, Example (13)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50520157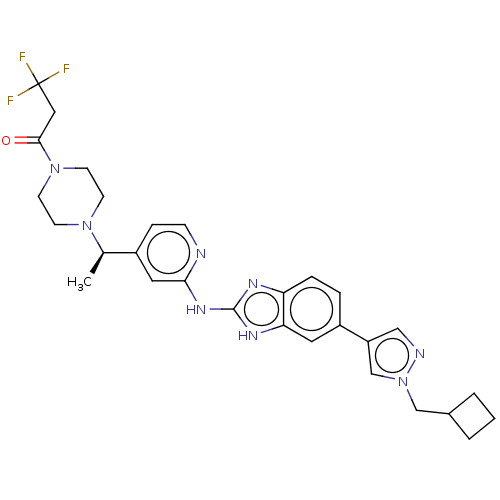 (CHEMBL4436188) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of wild-type human partial length DRAK1 (R32 to E363 residues) expressed in bacterial expression system by Kinomescan method | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50606011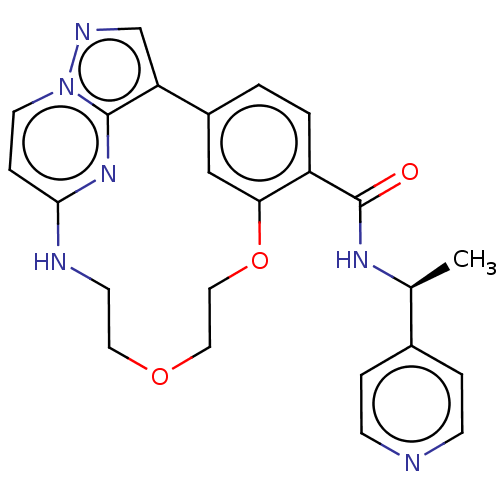 (CHEMBL5191892) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00173 BindingDB Entry DOI: 10.7270/Q2NC6587 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441876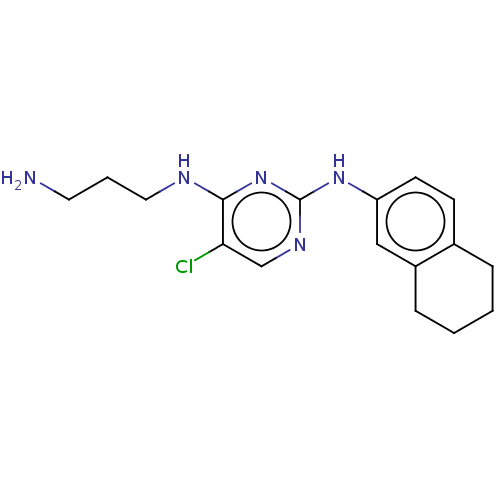 (US10647686, Example (23)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441873 (US10647686, Example (20)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441862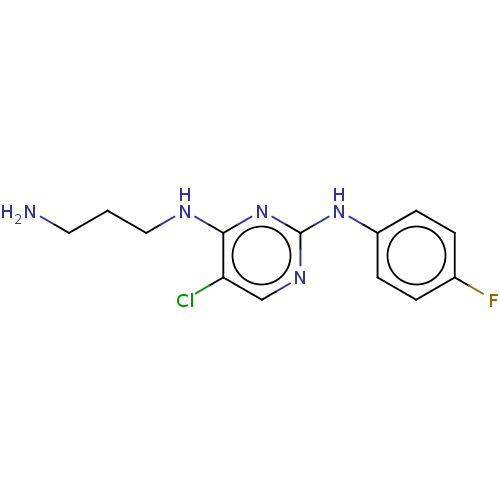 (US10647686, Example (9)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441882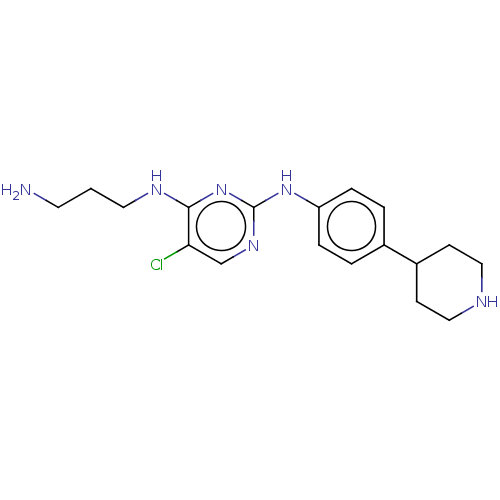 (US10647686, Example (26)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441878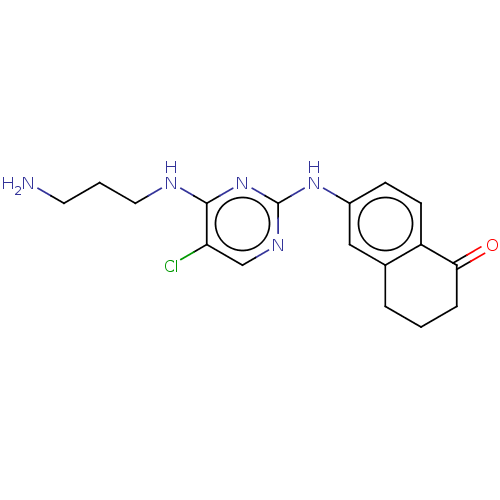 (US10647686, Example (24)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50166251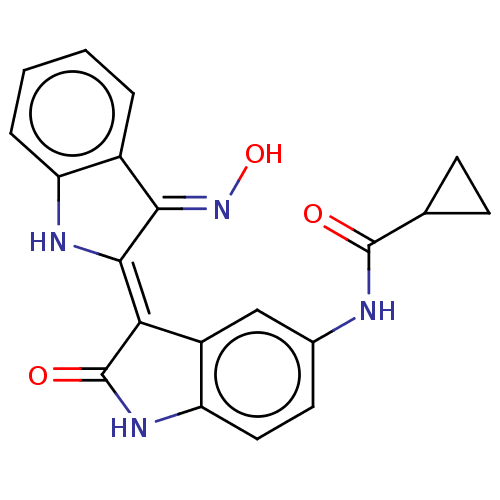 (CHEMBL3799389) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK1 (unknown origin) | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441874 (US10647686, Example (21)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441881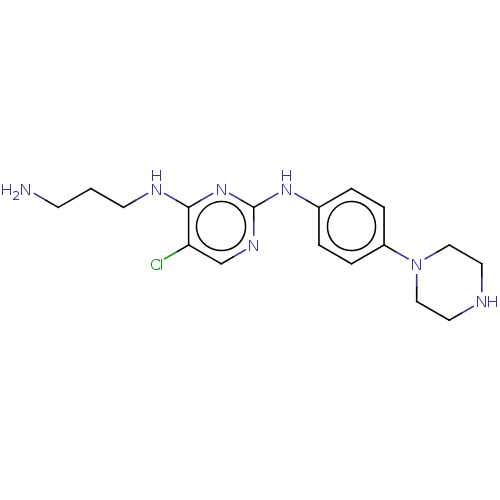 (US10647686, Example (25)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441872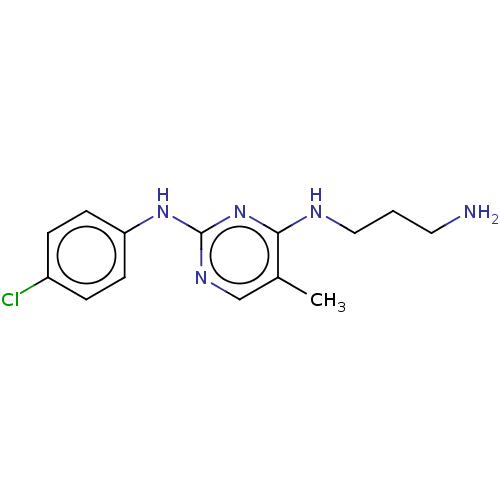 (US10647686, Example (19) | US10647686, Example 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441872 (US10647686, Example (19) | US10647686, Example 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441870 (US10647686, Example (17)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441872 (US10647686, Example (19) | US10647686, Example 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441858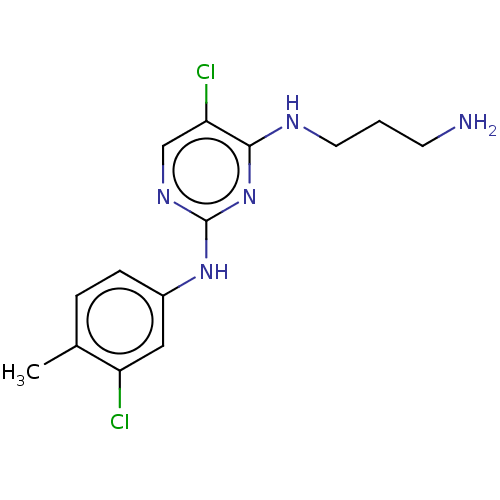 (US10647686, Example (6)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50166260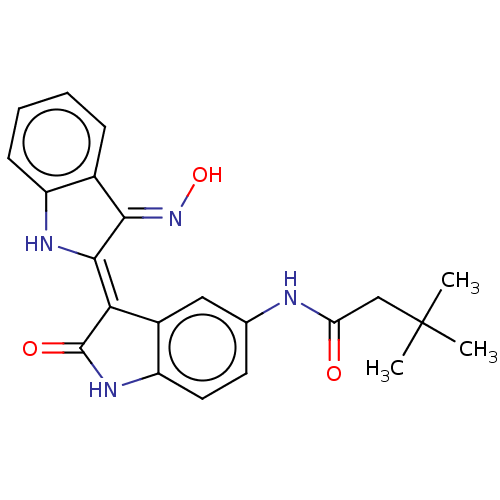 (CHEMBL3799505) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK1 (unknown origin) | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441875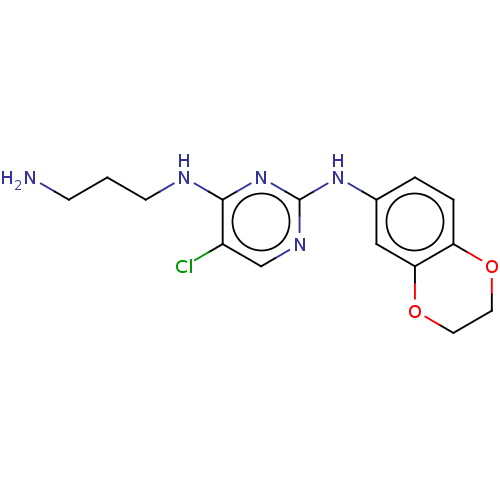 (US10647686, Example (22)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50166271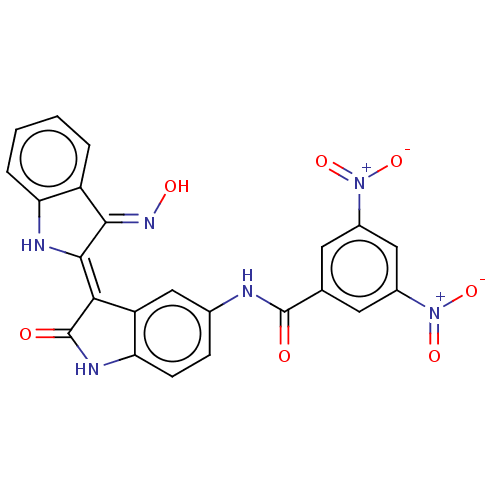 (CHEMBL3798563) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK1 (unknown origin) | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50520152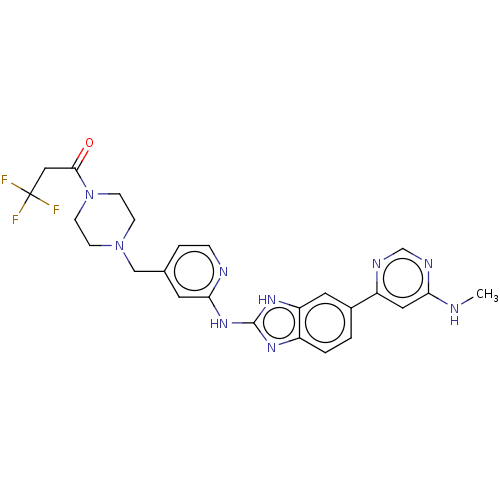 (CHEMBL4594167 | US10894784, Example 150.02) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of wild-type human partial length DRAK1 (R32 to E363 residues) expressed in bacterial expression system by Kinomescan method | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441863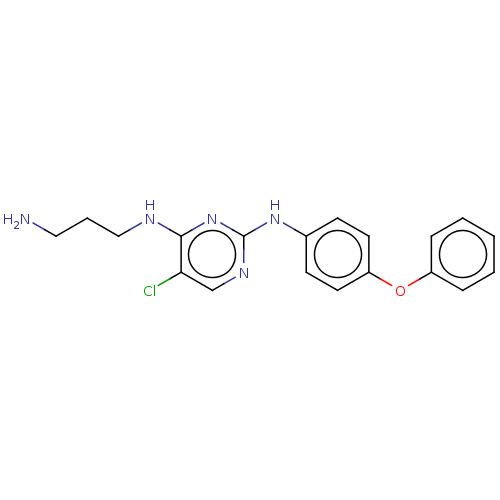 (US10647686, Example (10)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441854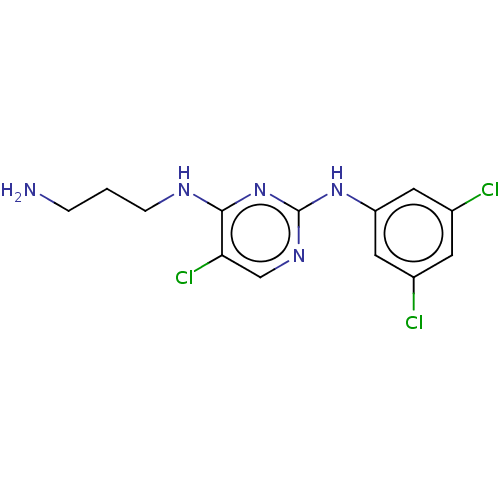 (US10647686, Example (2)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441868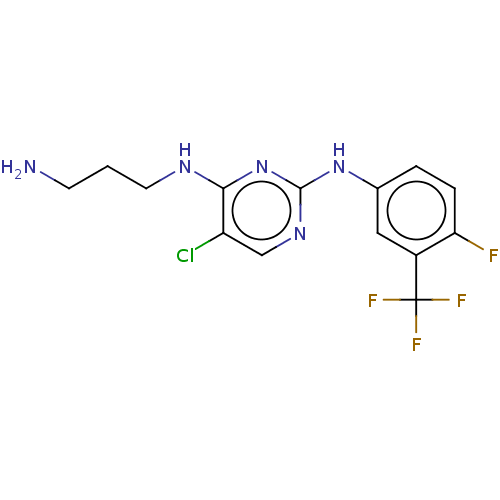 (US10647686, Example (15)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441904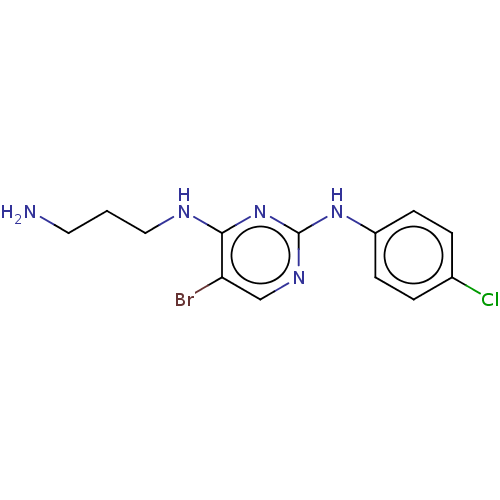 (US10647686, Comparative Example 6) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441904 (US10647686, Comparative Example 6) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50520150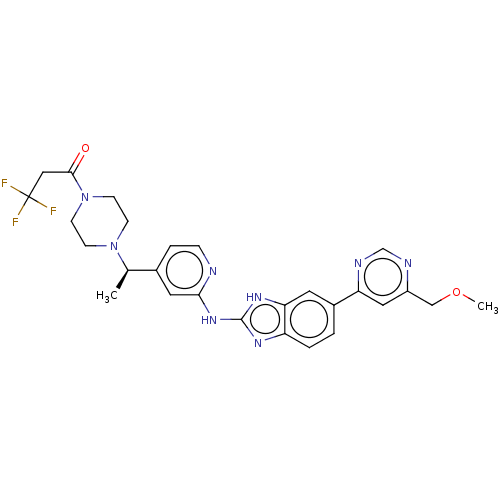 (CHEMBL4566796) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of wild-type human partial length DRAK1 (R32 to E363 residues) expressed in bacterial expression system by Kinomescan method | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human DRAK1 using KKLNRTLSFAEPG as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441898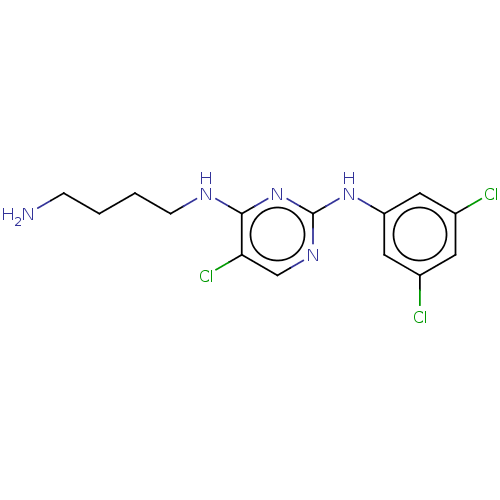 (US10647686, Example (41)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50606014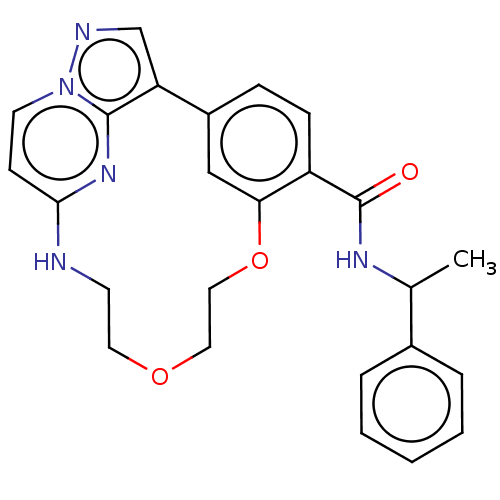 (CHEMBL5193635) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00173 BindingDB Entry DOI: 10.7270/Q2NC6587 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 31.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human DRAK1 using KKLNRTLSFAEPG as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50166077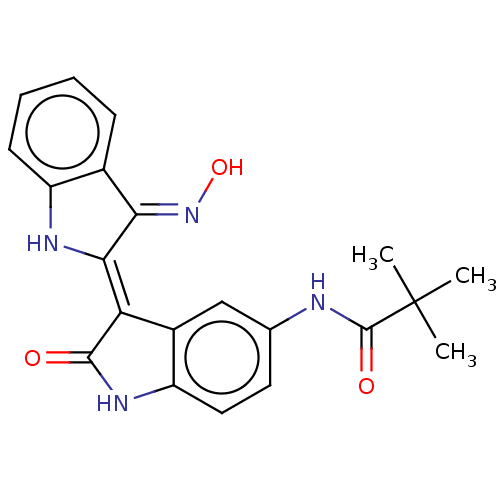 (CHEMBL513703) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK1 (unknown origin) | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441905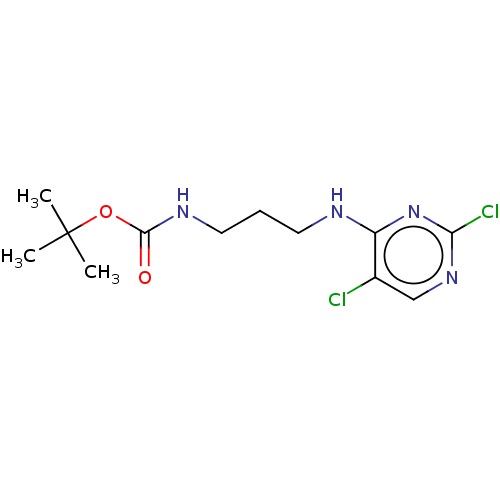 (US10647686, Example 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441869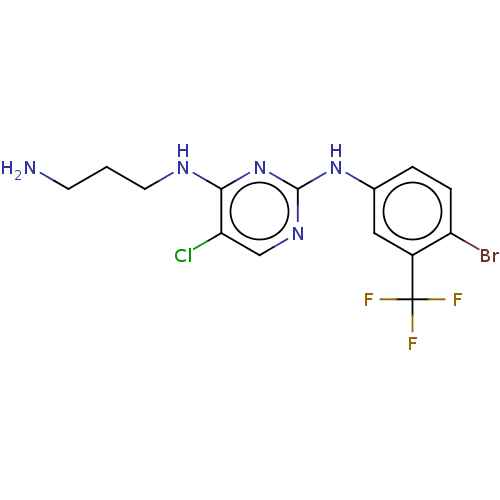 (US10647686, Example (16)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441895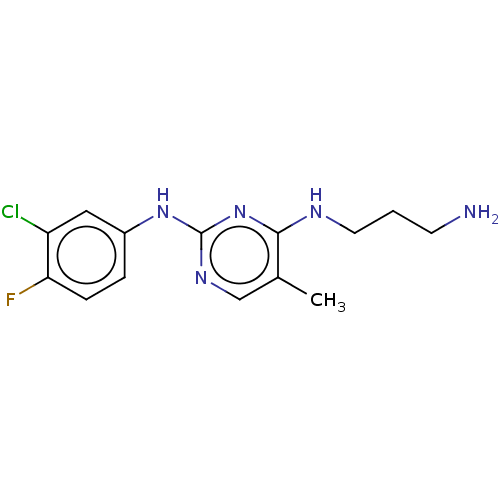 (US10647686, Example (38)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441871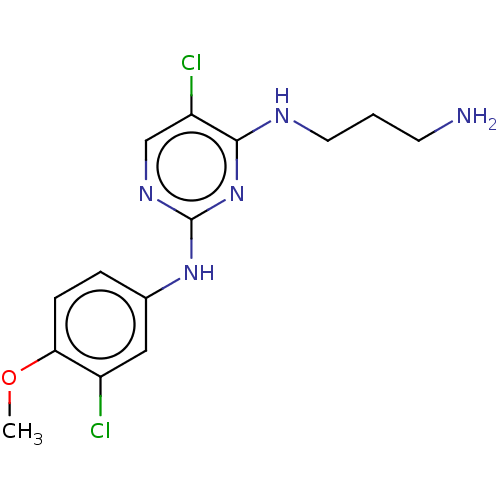 (US10647686, Example (18)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441867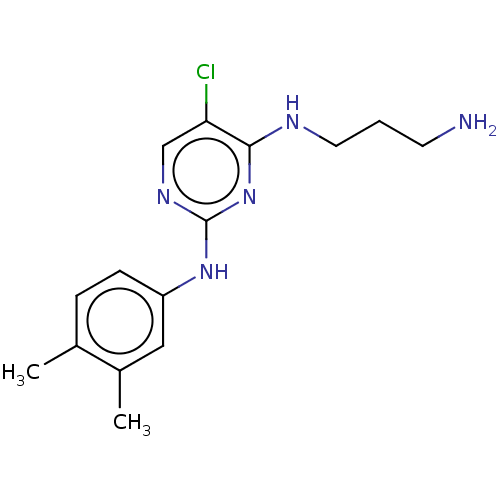 (US10647686, Example (14)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441905 (US10647686, Example 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441853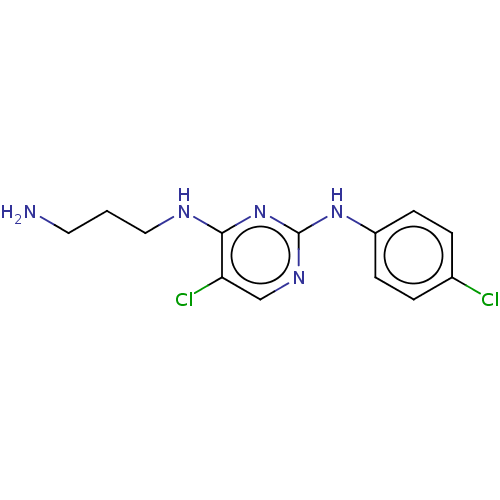 (US10647686, Example (1)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50111572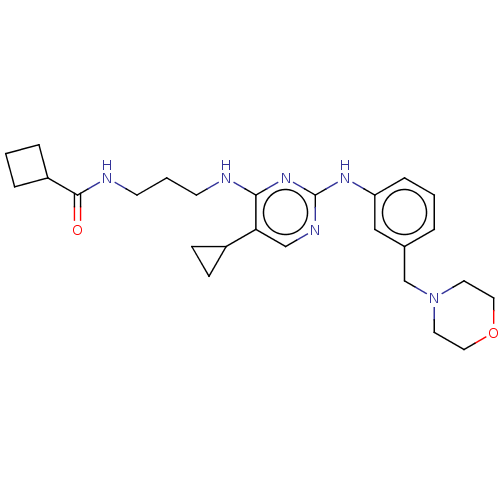 (CHEMBL3605057) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of tracer K9 from NLuc fused DRAK1 (unknown origin) expressed in HEK293 cells by NanoBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00440 BindingDB Entry DOI: 10.7270/Q27S7SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441864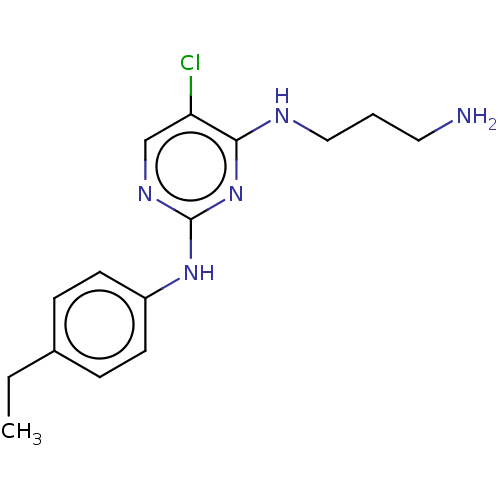 (US10647686, Example (11)) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441897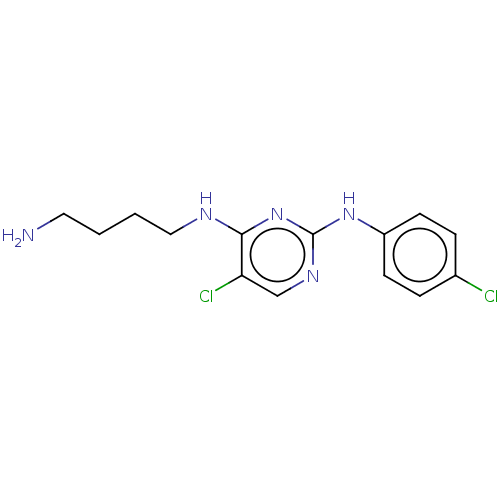 (US10647686, Example (40) | US10647686, Example 40) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM441897 (US10647686, Example (40) | US10647686, Example 40) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description To measure the DRAK1 and DRAK2 activity, the substrate MRCL3 peptide and ATP were mixed with the enzymes. After an appropriate period of time, the re... | US Patent US10647686 (2020) BindingDB Entry DOI: 10.7270/Q2MS3WSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50606012 (CHEMBL5087558) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00173 BindingDB Entry DOI: 10.7270/Q2NC6587 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM50166121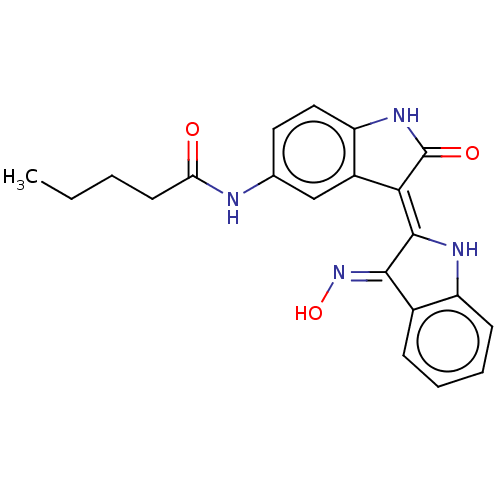 (CHEMBL3797480) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK1 (unknown origin) | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17A (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00173 BindingDB Entry DOI: 10.7270/Q2NC6587 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 153 total ) | Next | Last >> |