 Found 6 hits of ic50 data for polymerid = 4514
Found 6 hits of ic50 data for polymerid = 4514 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of ACTR-2B |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50466183

(CHEMBL4283638)Show SMILES CC(N1CCCC1)c1ccc(cc1)-c1cnc2c(cnn2c1)-c1ccc(c2ccccc12)S(N)(=O)=O Show InChI InChI=1S/C28H27N5O2S/c1-19(32-14-4-5-15-32)20-8-10-21(11-9-20)22-16-30-28-26(17-31-33(28)18-22)24-12-13-27(36(29,34)35)25-7-3-2-6-23(24)25/h2-3,6-13,16-19H,4-5,14-15H2,1H3,(H2,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human ACVR2B catalytic domain (185 to 488 residues) expressed in Baculovirus expression system by LanthaScreen assay |
Bioorg Med Chem Lett 28: 3356-3362 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.006
BindingDB Entry DOI: 10.7270/Q2NZ8BBT |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50183449
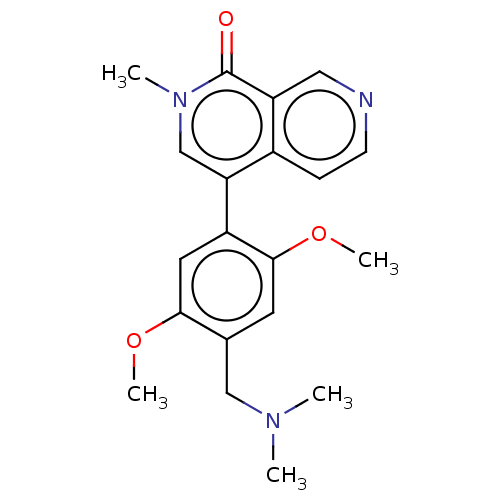
(CHEMBL3823101 | US11773085, Compound B23)Show SMILES COc1cc(c(OC)cc1CN(C)C)-c1cn(C)c(=O)c2cnccc12 Show InChI InChI=1S/C20H23N3O3/c1-22(2)11-13-8-19(26-5)15(9-18(13)25-4)17-12-23(3)20(24)16-10-21-7-6-14(16)17/h6-10,12H,11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of ACVR2B (unknown origin) |
J Med Chem 59: 4462-75 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01865
BindingDB Entry DOI: 10.7270/Q27H1MJZ |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50401152
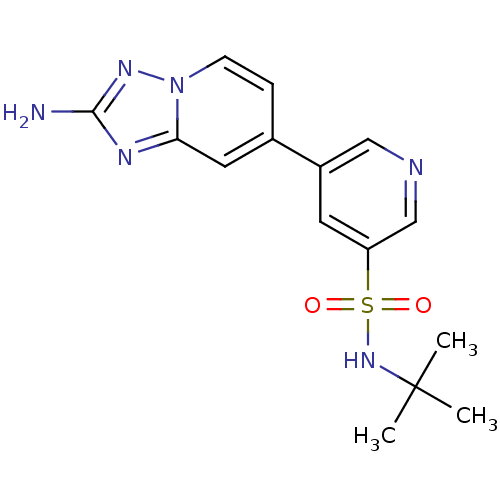
(CHEMBL2205766)Show SMILES CC(C)(C)NS(=O)(=O)c1cncc(c1)-c1ccn2nc(N)nc2c1 Show InChI InChI=1S/C15H18N6O2S/c1-15(2,3)20-24(22,23)12-6-11(8-17-9-12)10-4-5-21-13(7-10)18-14(16)19-21/h4-9,20H,1-3H3,(H2,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cellzome Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACVR2B |
Bioorg Med Chem Lett 22: 4613-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.090
BindingDB Entry DOI: 10.7270/Q2HQ412B |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50183448
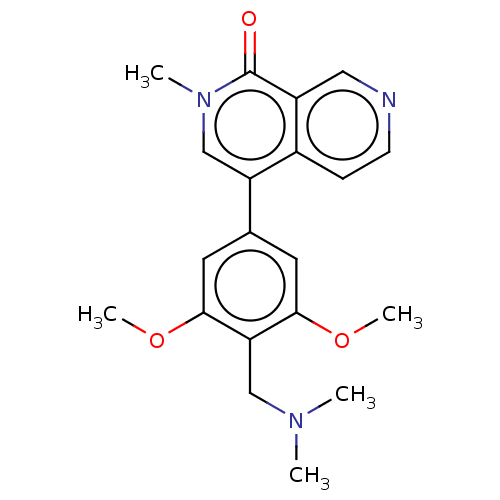
(CHEMBL3823478 | US11773085, Compound B2)Show SMILES COc1cc(cc(OC)c1CN(C)C)-c1cn(C)c(=O)c2cnccc12 Show InChI InChI=1S/C20H23N3O3/c1-22(2)11-17-18(25-4)8-13(9-19(17)26-5)16-12-23(3)20(24)15-10-21-7-6-14(15)16/h6-10,12H,11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim RCV GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of ACVR2B (unknown origin) |
J Med Chem 59: 4462-75 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01865
BindingDB Entry DOI: 10.7270/Q27H1MJZ |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50044849
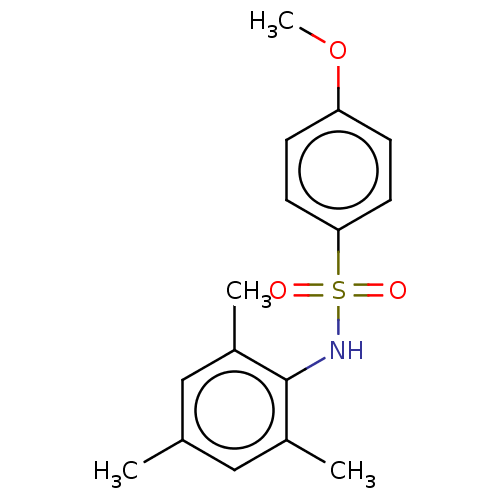
(CHEMBL3311308)Show InChI InChI=1S/C16H19NO3S/c1-11-9-12(2)16(13(3)10-11)17-21(18,19)15-7-5-14(20-4)6-8-15/h5-10,17H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human ACVR2B by Ant A204 luciferase reporter/summary (Abse5) assay |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data