

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin receptor (Mus musculus) | BDBM50459972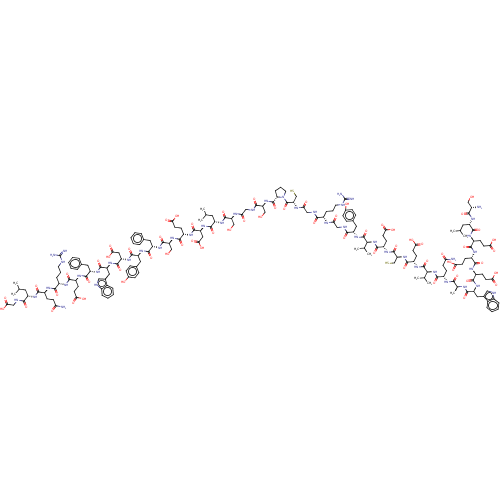 (CHEMBL4226003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Agonist activity at B6D2F1 mouse insulin receptor assessed as increase in glucose incorporation into lipid phase after 2 hrs in presence of D-[3-3H]g... | Bioorg Med Chem 26: 2827-2841 (2018) Article DOI: 10.1016/j.bmc.2017.09.030 BindingDB Entry DOI: 10.7270/Q2TX3J04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377967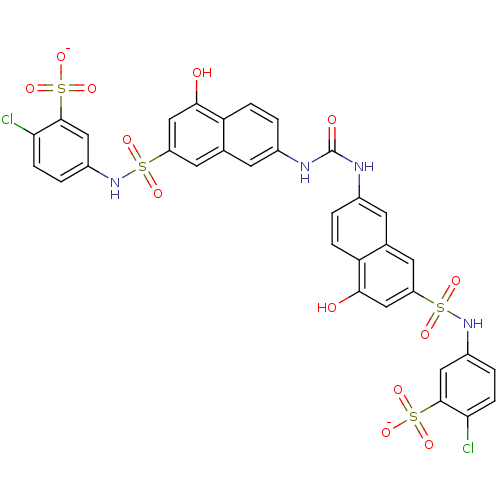 (CHEMBL1627107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253185 (2-Chloro-5-{[(7-{[(7-{[(4-chloro-3-sulfophenyl)ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253181 (2-Hydroxy-5-{[(7-{[(7-sulfo(2-naphthyl))amino]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253179 (5-{[(7-{[(7-{[(3-Carboxy-4-chlorophenyl)amino]sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253178 (5-{[(7-{[(7-{[(3-Carboxy-4-chlorophenyl)amino]sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377968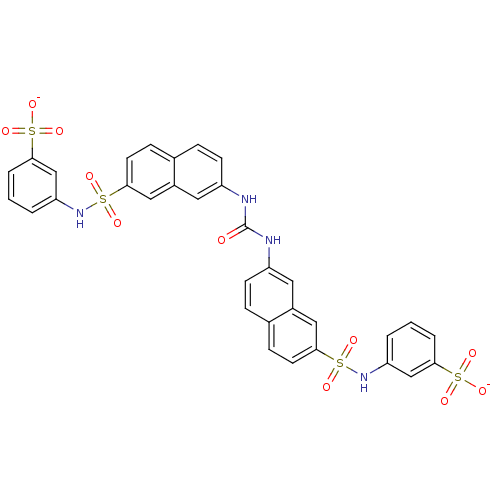 (CHEMBL1627106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253166 (3-{7-[({7-[(3-carboxyphenyl)sulfamoyl]naphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253159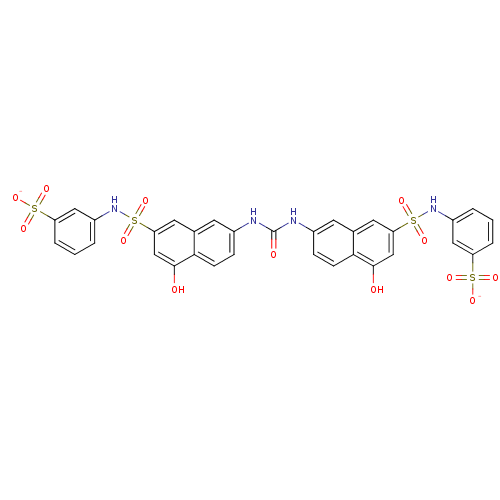 (3-{[(4-Hydroxy-7-{[(5-hydroxy-7-{[(3-sulfophenyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253182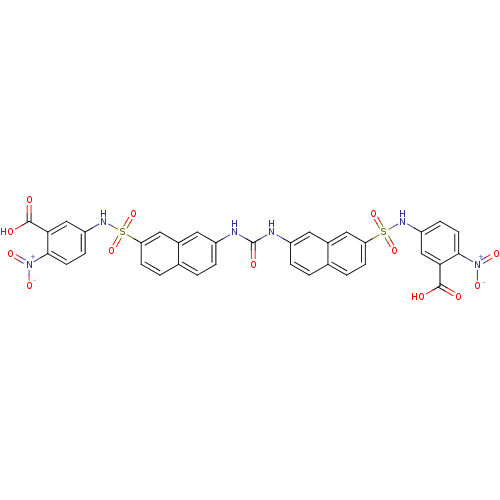 (5-{7-[({7-[(3-carboxy-4-nitrophenyl)sulfamoyl]naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253177 (2-{7-[({7-[(2-carboxyphenyl)sulfamoyl]naphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253165 (3-{7-[({7-[(3-carboxyphenyl)sulfamoyl]-5-hydroxyna...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253180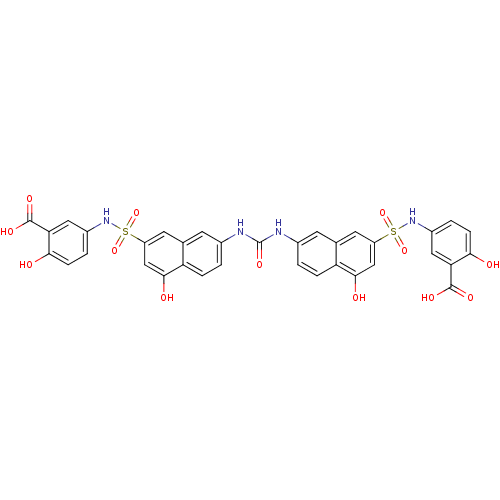 (5-{[(7-{[(7-{[(3-Carboxy-4-hydroxyphenyl)amino]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253168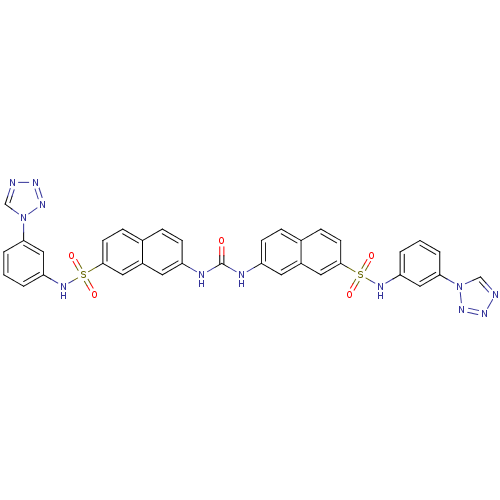 (1,3-bis(7-{[3-(1H-1,2,3,4-tetrazol-1-yl)phenyl]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253189 (5-[({7-[3-(7-{[(3-Carboxy-4-chlorophenyl)amino]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253167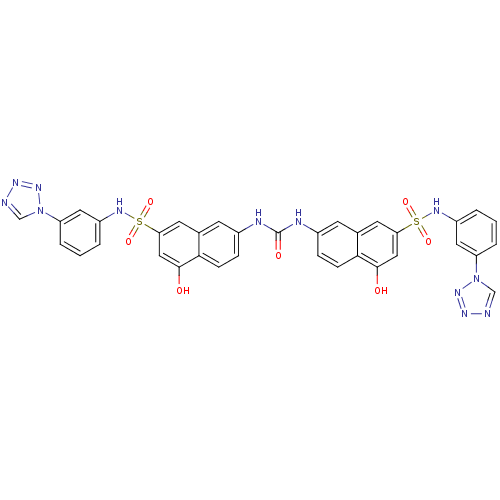 (1,3-bis(5-hydroxy-7-{[3-(1H-1,2,3,4-tetrazol-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377965 (CHEMBL436679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253192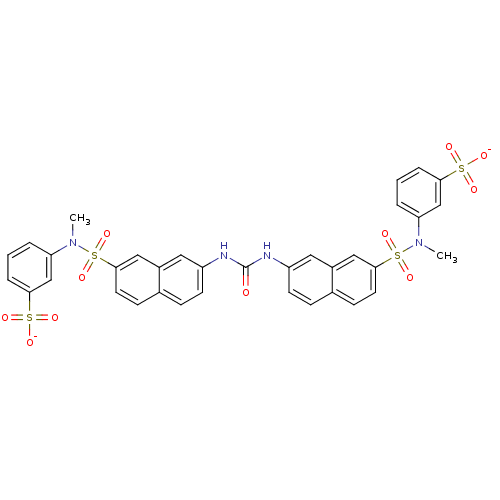 (CHEMBL505538 | disodium 3-{N-methyl7-[({7-[methyl(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253175 (2-{7-[({7-[(2-carboxyphenyl)sulfamoyl]-5-hydroxyna...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253173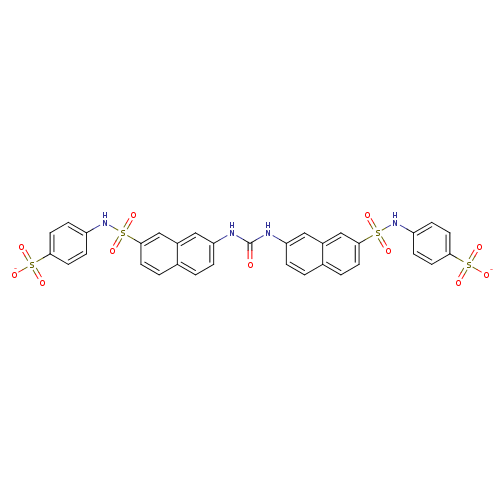 (CHEMBL499172 | disodium 4-{7-[({7-[(4-sulfonatophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253174 (4-{7-[({7-[(4-carboxyphenyl)sulfamoyl]-5-hydroxyna...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253184 (3-{7-[({7-[(3-carboxy-2-chlorophenyl)sulfamoyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253183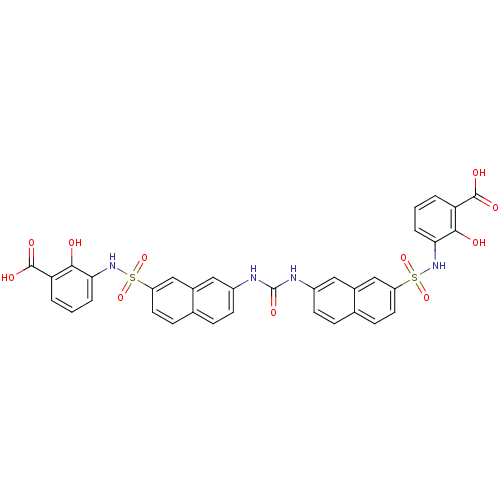 (3-{7-[({7-[(3-carboxy-2-hydroxyphenyl)sulfamoyl]na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253195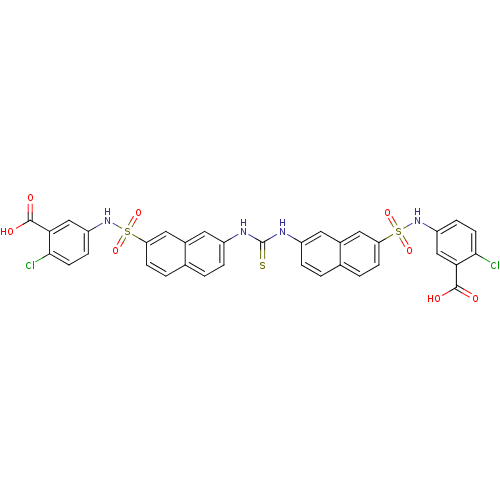 (5-({[7-({[(7-{[(3-Carboxy-4-chlorophenyl)amino]sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.62E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253196 (5-{[(7-{2-Amino-1-aza-2-[(7-{[(3-carboxy-4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253176 (4-{7-[({7-[(4-carboxyphenyl)sulfamoyl]naphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.83E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253164 (1,3-bis(5-hydroxy-7-{[3-(trifluoromethyl)phenyl]su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253163 (1,3-bis({7-[(3-cyanophenyl)sulfamoyl]naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253188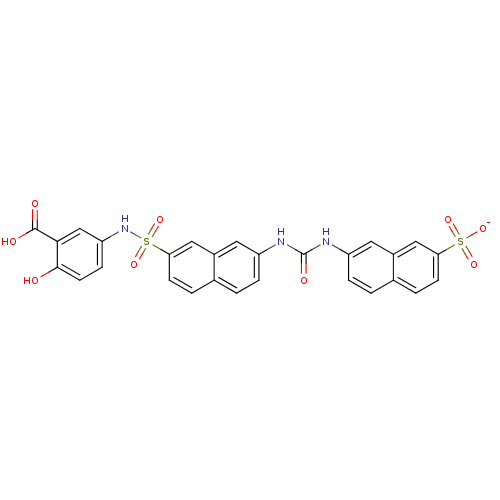 (CHEMBL453256 | sodium 7-[({7-[(3-carboxy-4-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253191 (CHEMBL442662 | disodium 3-{7-[({7-[(3-sulfonatophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253169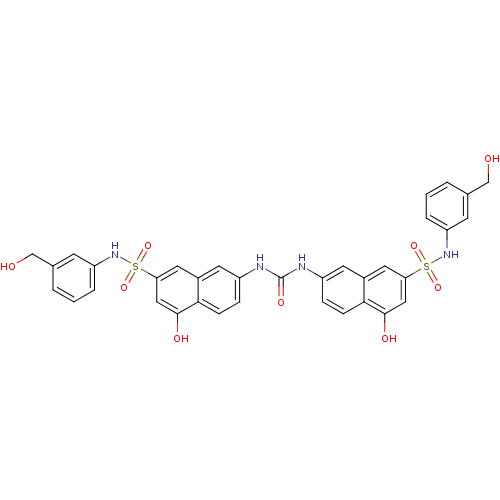 (1,3-bis(5-hydroxy-7-{[3-(hydroxymethyl)phenyl]sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253190 (3-({7-[(N-{7-[N-(3-Carboxyphenyl)carbamoyl]-2-naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253171 (1,3-bis({7-[(3-sulfamoylphenyl)sulfamoyl]naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253193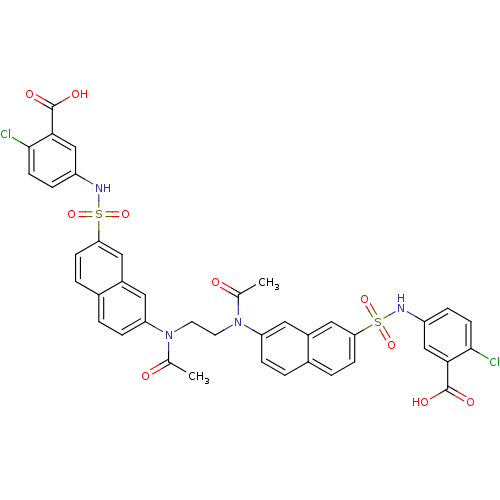 (5-({[7-(N-{2-[N-(7-{[(3-Carboxy-4-chlorophenyl)ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253170 (1,3-bis({7-[(3-hydroxyphenyl)sulfamoyl]naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253172 (2-[3-(7-{[(7-{[3-(carboxymethyl)phenyl]sulfamoyl}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253194 (5-({[7-({2-[(7-{[(3-Carboxy-4-chlorophenyl)amino]s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253199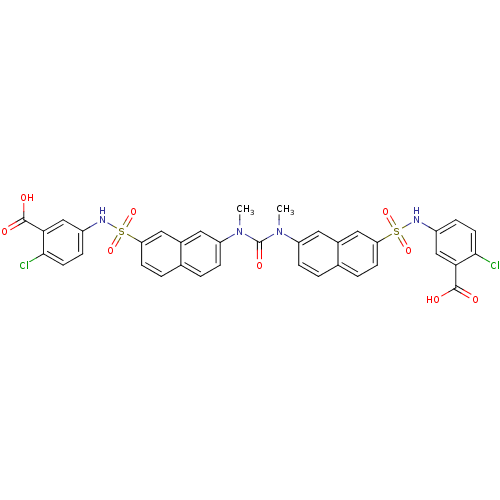 (5-{[(7-{[N-(7-{[(3-Carboxy-4-chlorophenyl)amino]su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253162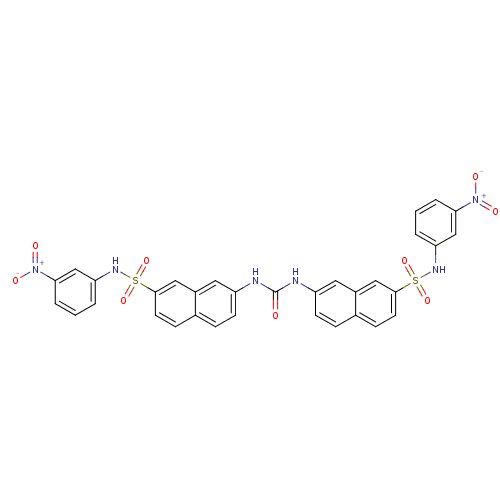 (1,3-bis({7-[(3-nitrophenyl)sulfamoyl]naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253161 (7,7'-carbonylbis(azanediyl)bis(N-phenylnaphthalene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253198 (5-[7-(1-{7-[(3-carboxy-4-chlorophenyl)sulfamoyl]na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253197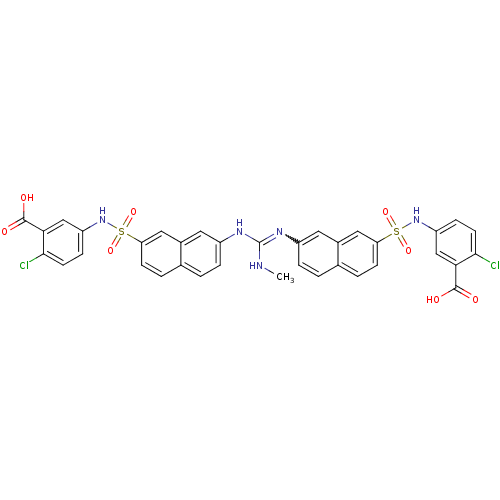 (5-[7-(1-{7-[(3-carboxy-4-chlorophenyl)sulfamoyl]na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377966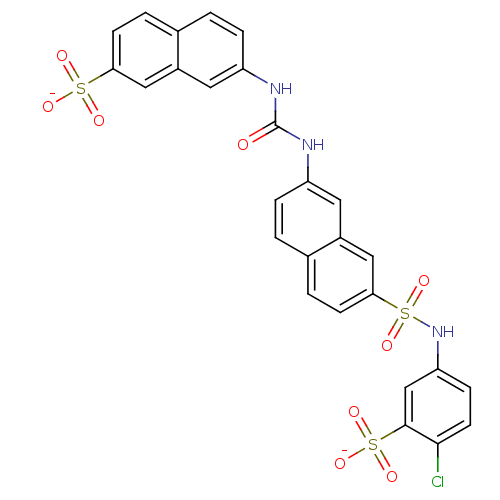 (CHEMBL1627108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||