 Found 21 hits of ic50 data for polymerid = 469,50000958
Found 21 hits of ic50 data for polymerid = 469,50000958 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin receptor
(Mus musculus) | BDBM50296348
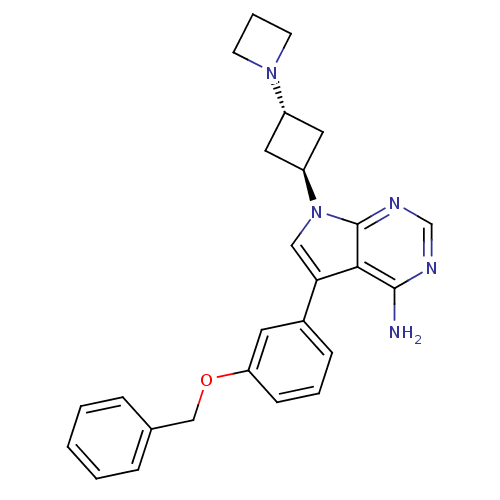
(7-(3-(azetidin-1-yl)cyclobutyl)-5-(3-(benzyloxy)ph...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCC1 |r,wU:26.32,wD:24.27,(12.58,1.25,;12.59,-.29,;11.26,-1.07,;11.26,-2.61,;12.59,-3.38,;13.92,-2.61,;15.4,-3.09,;16.31,-1.83,;15.4,-.58,;15.87,.89,;14.85,2.02,;15.32,3.49,;16.83,3.81,;17.87,2.67,;19.37,2.99,;20.4,1.84,;21.91,2.16,;22.94,1.01,;24.44,1.33,;24.92,2.8,;23.88,3.95,;22.38,3.62,;17.39,1.2,;13.92,-1.06,;15.87,-4.56,;15.17,-5.92,;16.54,-6.63,;17.25,-5.26,;17.01,-8.1,;16.3,-9.45,;17.67,-10.16,;18.37,-8.79,)| Show InChI InChI=1S/C26H27N5O/c27-25-24-23(19-8-4-9-22(12-19)32-16-18-6-2-1-3-7-18)15-31(26(24)29-17-28-25)21-13-20(14-21)30-10-5-11-30/h1-4,6-9,12,15,17,20-21H,5,10-11,13-14,16H2,(H2,27,28,29)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor-mediated proliferation of mouse NIH/3T3 cells after 48 hrs by MTT assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Rattus norvegicus (rat)) | BDBM482158
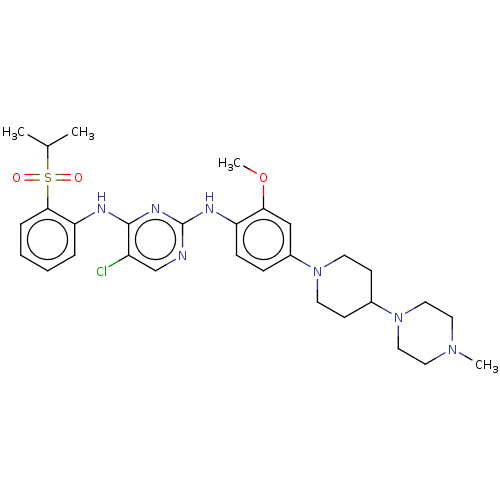
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of InsR phosphorylation in rat H4-2E cells |
Proc Natl Acad Sci U S A 104: 270-5 (2007)
Article DOI: 10.1073/pnas.0609412103
BindingDB Entry DOI: 10.7270/Q2BP02K2 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50384721
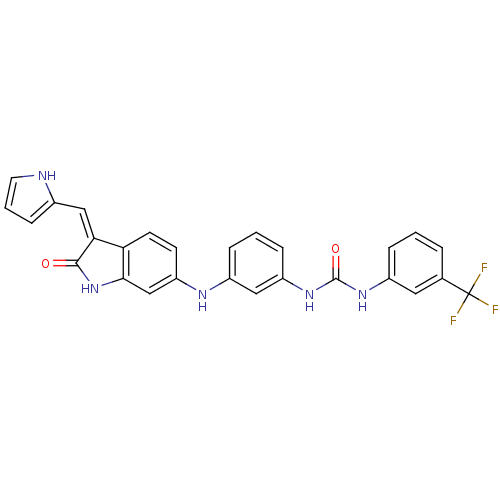
(CHEMBL2037224)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2cccc(Nc3ccc4\C(=C\c5ccc[nH]5)C(=O)Nc4c3)c2)c1 Show InChI InChI=1S/C27H20F3N5O2/c28-27(29,30)16-4-1-5-18(12-16)33-26(37)34-20-7-2-6-19(13-20)32-21-9-10-22-23(14-17-8-3-11-31-17)25(36)35-24(22)15-21/h1-15,31-32H,(H,35,36)(H2,33,34,37)/b23-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused INSR in mouse BA/F3 cells |
ACS Med Chem Lett 3: 140-145 (2012)
Article DOI: 10.1021/ml200261d
BindingDB Entry DOI: 10.7270/Q2SJ1MNM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50384734
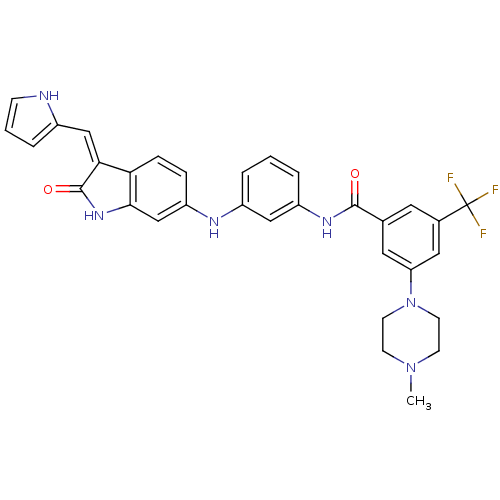
(CHEMBL2037211)Show SMILES CN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1cccc(Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)c1 Show InChI InChI=1S/C32H29F3N6O2/c1-40-10-12-41(13-11-40)26-15-20(14-21(16-26)32(33,34)35)30(42)38-24-5-2-4-23(17-24)37-25-7-8-27-28(18-22-6-3-9-36-22)31(43)39-29(27)19-25/h2-9,14-19,36-37H,10-13H2,1H3,(H,38,42)(H,39,43)/b28-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused INSR in mouse BA/F3 cells |
ACS Med Chem Lett 3: 140-145 (2012)
Article DOI: 10.1021/ml200261d
BindingDB Entry DOI: 10.7270/Q2SJ1MNM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50384725
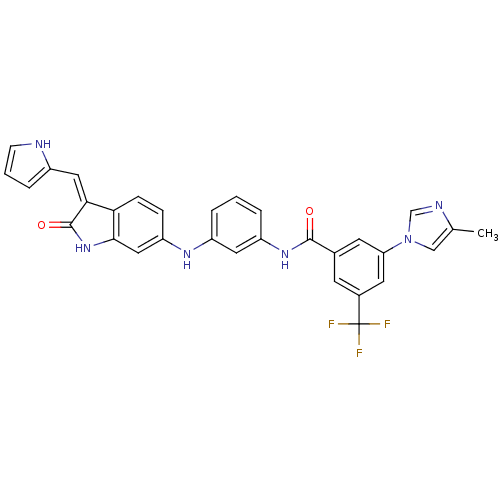
(CHEMBL2037209)Show SMILES Cc1cn(cn1)-c1cc(cc(c1)C(F)(F)F)C(=O)Nc1cccc(Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)c1 Show InChI InChI=1S/C31H23F3N6O2/c1-18-16-40(17-36-18)25-11-19(10-20(12-25)31(32,33)34)29(41)38-23-5-2-4-22(13-23)37-24-7-8-26-27(14-21-6-3-9-35-21)30(42)39-28(26)15-24/h2-17,35,37H,1H3,(H,38,41)(H,39,42)/b27-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused INSR in mouse BA/F3 cells |
ACS Med Chem Lett 3: 140-145 (2012)
Article DOI: 10.1021/ml200261d
BindingDB Entry DOI: 10.7270/Q2SJ1MNM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50384726
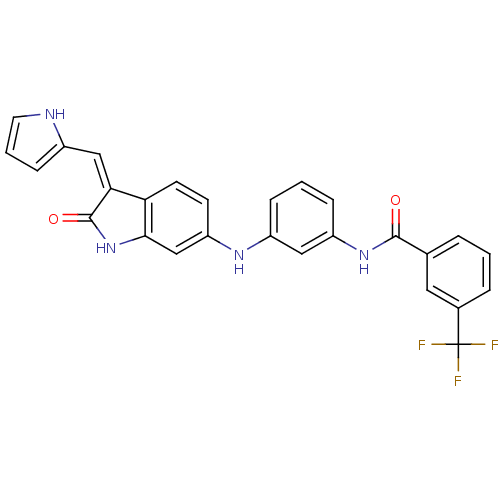
(CHEMBL2037208)Show SMILES FC(F)(F)c1cccc(c1)C(=O)Nc1cccc(Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)c1 Show InChI InChI=1S/C27H19F3N4O2/c28-27(29,30)17-5-1-4-16(12-17)25(35)33-20-7-2-6-19(13-20)32-21-9-10-22-23(14-18-8-3-11-31-18)26(36)34-24(22)15-21/h1-15,31-32H,(H,33,35)(H,34,36)/b23-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused INSR in mouse BA/F3 cells |
ACS Med Chem Lett 3: 140-145 (2012)
Article DOI: 10.1021/ml200261d
BindingDB Entry DOI: 10.7270/Q2SJ1MNM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50066982
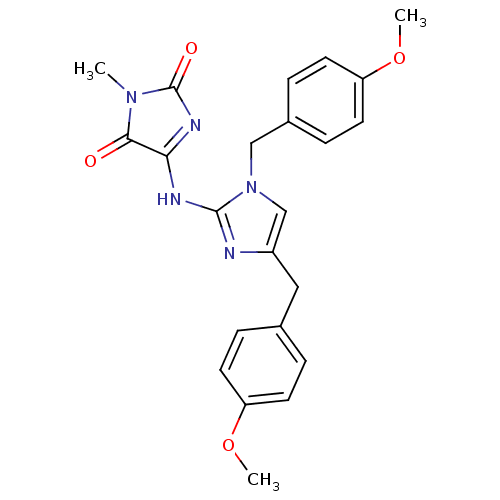
(5-[1,4-Bis-(4-methoxy-benzyl)-1H-imidazol-2-ylamin...)Show SMILES COc1ccc(Cc2cn(Cc3ccc(OC)cc3)c(NC3=NC(=O)N(C)C3=O)n2)cc1 |t:22| Show InChI InChI=1S/C23H23N5O4/c1-27-21(29)20(26-23(27)30)25-22-24-17(12-15-4-8-18(31-2)9-5-15)14-28(22)13-16-6-10-19(32-3)11-7-16/h4-11,14H,12-13H2,1-3H3,(H,24,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor mediated mitogenesis of NIH3T3 cells |
J Med Chem 41: 3909-11 (1998)
Article DOI: 10.1021/jm980294n
BindingDB Entry DOI: 10.7270/Q25H7FDR |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50384727
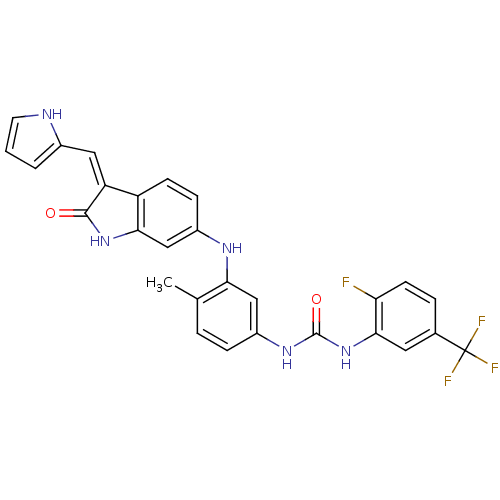
(CHEMBL2037226)Show SMILES Cc1ccc(NC(=O)Nc2cc(ccc2F)C(F)(F)F)cc1Nc1ccc2\C(=C\c3ccc[nH]3)C(=O)Nc2c1 Show InChI InChI=1S/C28H21F4N5O2/c1-15-4-6-19(35-27(39)37-25-11-16(28(30,31)32)5-9-22(25)29)13-23(15)34-18-7-8-20-21(12-17-3-2-10-33-17)26(38)36-24(20)14-18/h2-14,33-34H,1H3,(H,36,38)(H2,35,37,39)/b21-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused INSR in mouse BA/F3 cells |
ACS Med Chem Lett 3: 140-145 (2012)
Article DOI: 10.1021/ml200261d
BindingDB Entry DOI: 10.7270/Q2SJ1MNM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50066983

(5-[1-(4-Hydroxy-benzyl)-4-(4-methoxy-benzyl)-1H-im...)Show SMILES COc1ccc(Cc2cn(Cc3ccc(O)cc3)c(NC3=NC(=O)N(C)C3=O)n2)cc1 |t:21| Show InChI InChI=1S/C22H21N5O4/c1-26-20(29)19(25-22(26)30)24-21-23-16(11-14-5-9-18(31-2)10-6-14)13-27(21)12-15-3-7-17(28)8-4-15/h3-10,13,28H,11-12H2,1-2H3,(H,23,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor mediated mitogenesis of NIH3T3 cells |
J Med Chem 41: 3909-11 (1998)
Article DOI: 10.1021/jm980294n
BindingDB Entry DOI: 10.7270/Q25H7FDR |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50162993
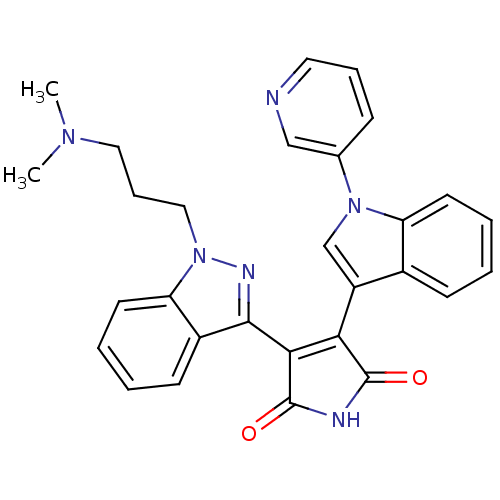
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccnc3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H26N6O2/c1-33(2)15-8-16-35-24-13-6-4-11-21(24)27(32-35)26-25(28(36)31-29(26)37)22-18-34(19-9-7-14-30-17-19)23-12-5-3-10-20(22)23/h3-7,9-14,17-18H,8,15-16H2,1-2H3,(H,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50162996
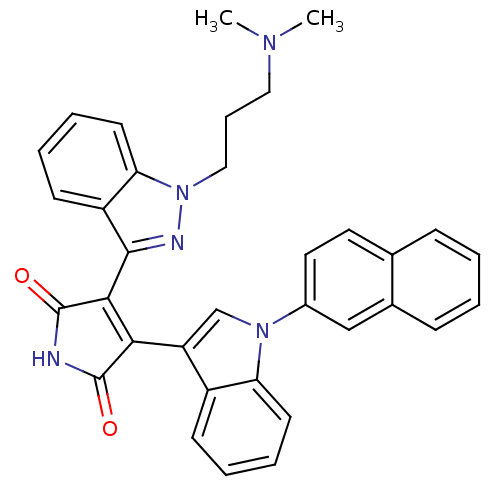
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3ccc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)18-9-19-39-29-15-8-6-13-26(29)32(36-39)31-30(33(40)35-34(31)41)27-21-38(28-14-7-5-12-25(27)28)24-17-16-22-10-3-4-11-23(22)20-24/h3-8,10-17,20-21H,9,18-19H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50162990
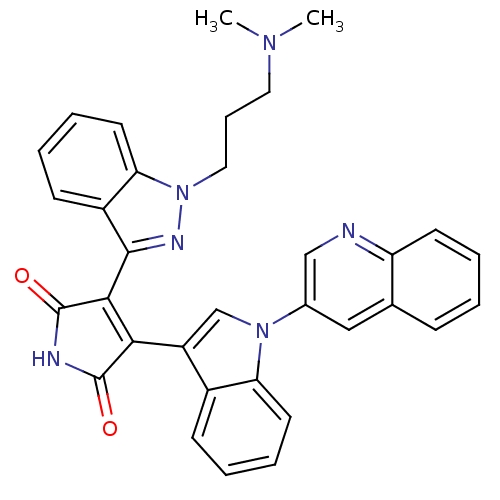
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cnc4ccccc4c3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C33H28N6O2/c1-37(2)16-9-17-39-28-15-8-5-12-24(28)31(36-39)30-29(32(40)35-33(30)41)25-20-38(27-14-7-4-11-23(25)27)22-18-21-10-3-6-13-26(21)34-19-22/h3-8,10-15,18-20H,9,16-17H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50384720
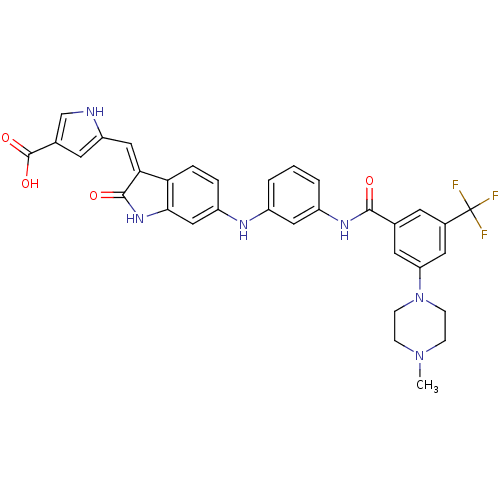
(CHEMBL2037220)Show SMILES CN1CCN(CC1)c1cc(cc(c1)C(F)(F)F)C(=O)Nc1cccc(Nc2ccc3\C(=C\c4cc(c[nH]4)C(O)=O)C(=O)Nc3c2)c1 Show InChI InChI=1S/C33H29F3N6O4/c1-41-7-9-42(10-8-41)26-13-19(11-21(14-26)33(34,35)36)30(43)39-23-4-2-3-22(15-23)38-24-5-6-27-28(31(44)40-29(27)17-24)16-25-12-20(18-37-25)32(45)46/h2-6,11-18,37-38H,7-10H2,1H3,(H,39,43)(H,40,44)(H,45,46)/b28-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tel-fused INSR in mouse BA/F3 cells |
ACS Med Chem Lett 3: 140-145 (2012)
Article DOI: 10.1021/ml200261d
BindingDB Entry DOI: 10.7270/Q2SJ1MNM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50162994
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-[...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C34H29N5O2/c1-37(2)19-10-20-39-29-17-8-6-15-25(29)32(36-39)31-30(33(40)35-34(31)41)26-21-38(28-16-7-5-14-24(26)28)27-18-9-12-22-11-3-4-13-23(22)27/h3-9,11-18,21H,10,19-20H2,1-2H3,(H,35,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50162998
(3-[1-(3-Dimethylamino-propyl)-1H-indazol-3-yl]-4-(...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3cccs3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C28H25N5O2S/c1-31(2)14-8-15-33-22-12-6-4-10-19(22)26(30-33)25-24(27(34)29-28(25)35)20-17-32(23-13-7-16-36-23)21-11-5-3-9-18(20)21/h3-7,9-13,16-17H,8,14-15H2,1-2H3,(H,29,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50162991

(3-(1-Benzo[b]thiophen-2-yl-1H-indol-3-yl)-4-[1-(3-...)Show SMILES CN(C)CCCn1nc(C2=C(C(=O)NC2=O)c2cn(-c3csc4ccccc34)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C32H27N5O2S/c1-35(2)16-9-17-37-25-14-7-4-12-22(25)30(34-37)29-28(31(38)33-32(29)39)23-18-36(24-13-6-3-10-20(23)24)26-19-40-27-15-8-5-11-21(26)27/h3-8,10-15,18-19H,9,16-17H2,1-2H3,(H,33,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor kinase in P19 cells |
J Med Chem 48: 1725-8 (2005)
Article DOI: 10.1021/jm049478u
BindingDB Entry DOI: 10.7270/Q2765DVX |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50271906
(2-benzyl-N4-(3-bromophenyl)-1H-indole-4,6-diamine ...)Show SMILES Nc1nc(Nc2cccc(Br)c2)c2cc(Cc3ccccc3)[nH]c2n1 Show InChI InChI=1S/C19H16BrN5/c20-13-7-4-8-14(10-13)22-17-16-11-15(9-12-5-2-1-3-6-12)23-18(16)25-19(21)24-17/h1-8,10-11H,9H2,(H4,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor-mediated proliferation of mouse NIH/3T3 cells after 48 hrs by MTT assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50271907
(2-(2-chlorobenzyl)-N4-(3-bromophenyl)-1H-indole-4,...)Show SMILES Nc1nc(Nc2cccc(Br)c2)c2cc(Cc3ccccc3Cl)[nH]c2n1 Show InChI InChI=1S/C19H15BrClN5/c20-12-5-3-6-13(9-12)23-17-15-10-14(24-18(15)26-19(22)25-17)8-11-4-1-2-7-16(11)21/h1-7,9-10H,8H2,(H4,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor-mediated proliferation of mouse NIH/3T3 cells after 48 hrs by MTT assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Rattus norvegicus (rat)) | BDBM2683
(2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Laboratoires Glaxo
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] lab... |
J Biol Chem 266: 15771-81 (1991)
BindingDB Entry DOI: 10.7270/Q2FF3QJ4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50066984

(4-(5-(4-hydroxybenzyl)-4-(4-methoxybenzyl)-1-methy...)Show SMILES COc1ccc(Cc2nc(NC3=NC(=O)N(C)C3=O)n(C)c2Cc2ccc(O)cc2)cc1 |t:11| Show InChI InChI=1S/C23H23N5O4/c1-27-19(13-15-4-8-16(29)9-5-15)18(12-14-6-10-17(32-3)11-7-14)24-22(27)25-20-21(30)28(2)23(31)26-20/h4-11,29H,12-13H2,1-3H3,(H,24,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor mediated mitogenesis of NIH3T3 cells |
J Med Chem 41: 3909-11 (1998)
Article DOI: 10.1021/jm980294n
BindingDB Entry DOI: 10.7270/Q25H7FDR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data