

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50525490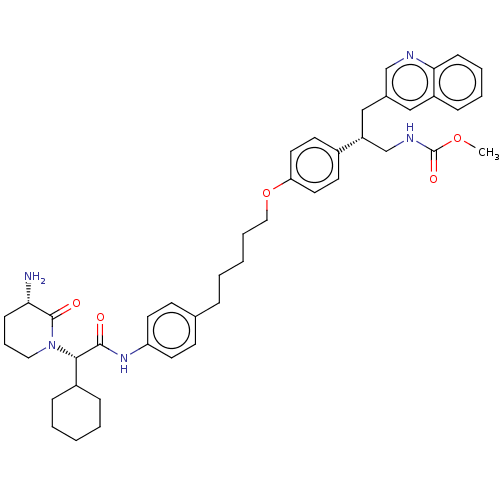 (CHEMBL4437643) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of recombinant IDE exosite (unknown origin) expressed in Escherichia coli using insulin as substrate incubated for 4 hrs by AlphaLisa assa... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using insulin as substrate preincubated for 10 mins followed by su... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using ATTO 655- Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substra... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50525489 (CHEMBL4556893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of IDE (unknown origin) assessed as cleavage of Mca-RPPGFSAFK(Dnp)-OH by fluorescence assay | J Med Chem 59: 6629-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01874 BindingDB Entry DOI: 10.7270/Q2N58QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50525489 (CHEMBL4556893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of IDE (unknown origin) assessed as cleavage of Mca-RPPGFSAFK(Dnp)-OH by fluorescence assay | J Med Chem 59: 6629-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01874 BindingDB Entry DOI: 10.7270/Q2N58QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209049 (US9243038, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50462574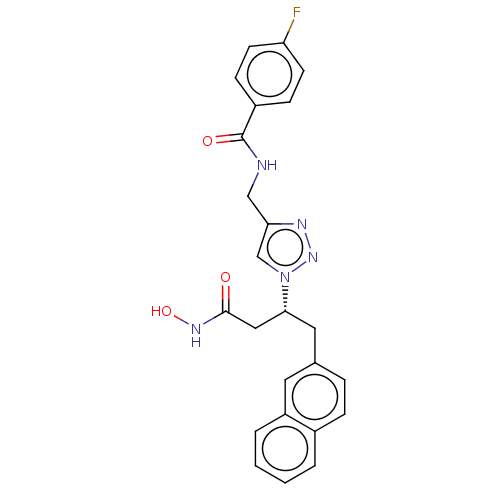 (CHEMBL4241044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of human IDE using insulin as substrate preincubated for 10 mis followed by substrate addition and measured after 30 mins | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50514415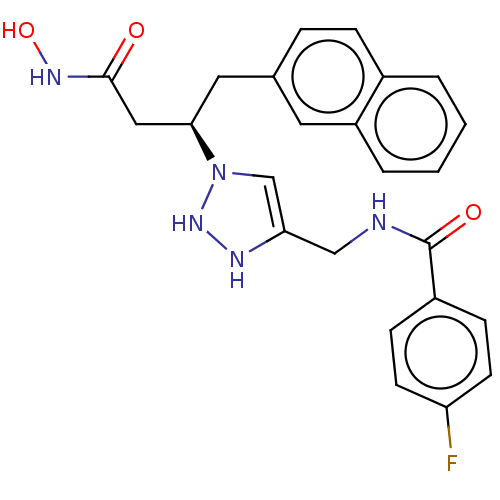 (CHEMBL4527561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE pre-incubated for 10 mins before Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp substrate addition and measured after 30... | J Med Chem 63: 3817-3833 (2020) Article DOI: 10.1021/acs.jmedchem.9b01183 BindingDB Entry DOI: 10.7270/Q2NZ8C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50462574 (CHEMBL4241044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using insulin as substrate preincubated for 10 mins followed by su... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209041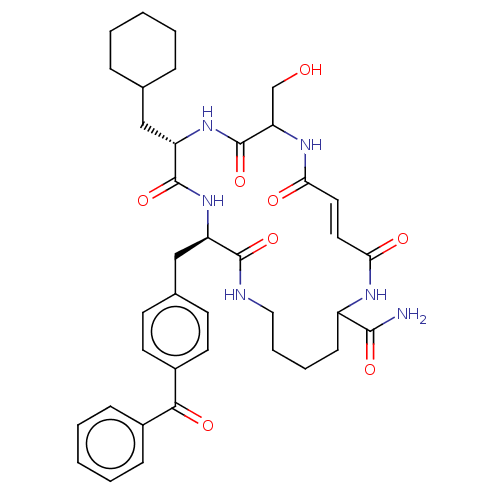 (US9243038, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209054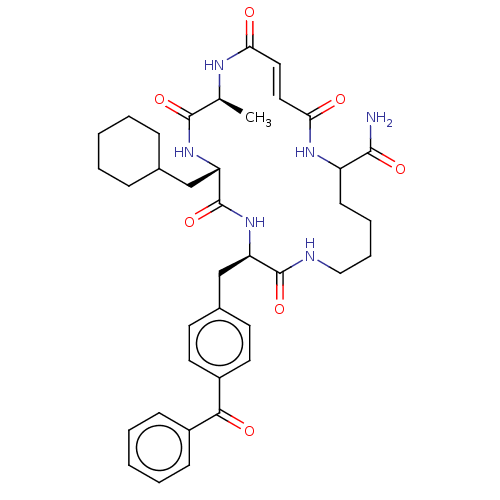 (US9243038, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209053 (US9243038, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209032 (US9243038, 6a (cis olefin) | US9243038, 6b (trans ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209051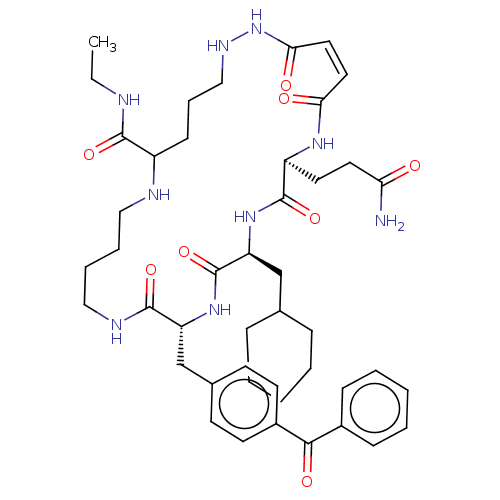 (US9243038, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM314096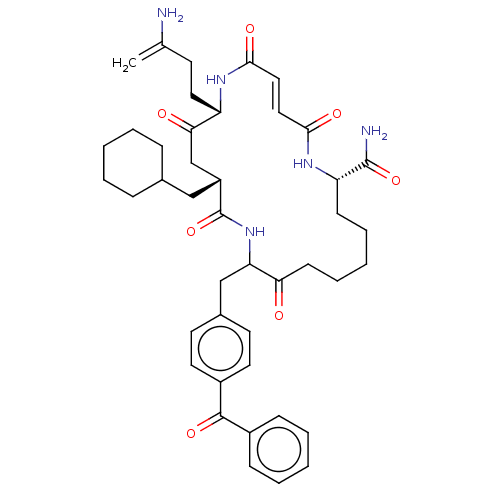 (US9610322, 6b (trans olefin)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description In vitro protease assays: Protease assays with IDE and neprilysin were performed using Mca-RPPGFSAFK(Dnp)-OH substrate peptide (R&D Systems, SEQ ID N... | US Patent US9610322 (2017) BindingDB Entry DOI: 10.7270/Q29Z970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50462574 (CHEMBL4241044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of wild type human IDE catalytic site using insulin as substrate preincubated for 10 mins followed by substrate addition and measured afte... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209045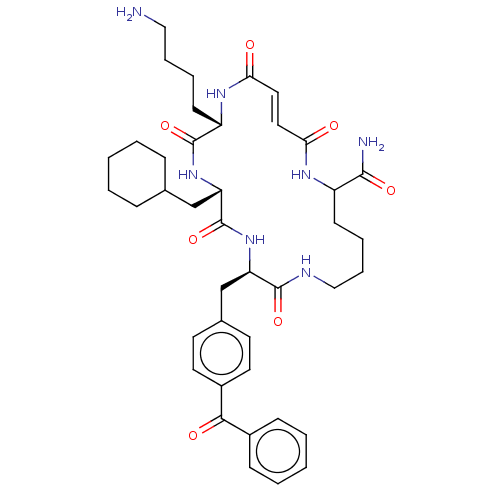 (US9243038, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209048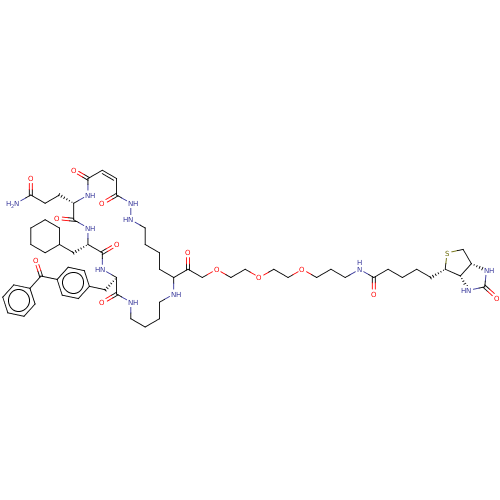 (US9243038, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209046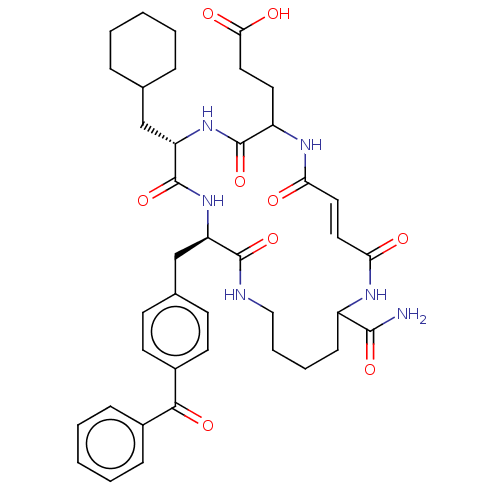 (US9243038, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005641 (CHEMBL3235416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209052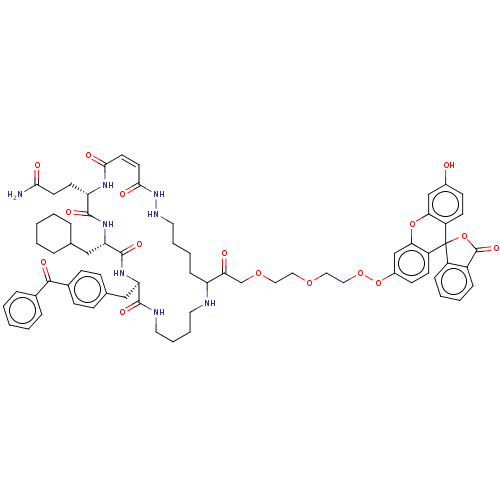 (US9243038, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005641 (CHEMBL3235416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of IDE exosite and catalytic site (unknown origin) | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209024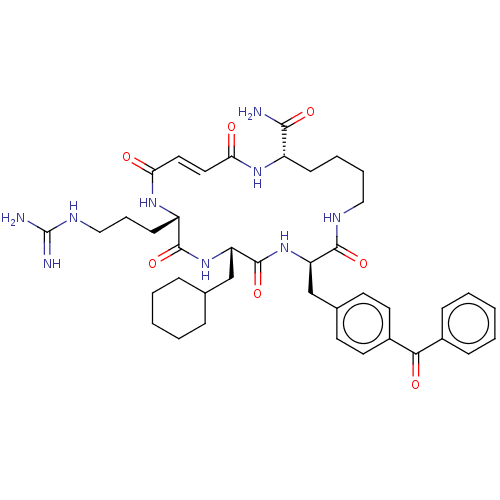 (US9243038, 2a (cis olefin) | US9243038, 2b (trans ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50462574 (CHEMBL4241044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE harboring C110L/C171S/C178A/C257V/C414L/C573N/C590S/C789S/C812A/C819A/C904S/C966N/C974A mutant expressed in Esche... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209042 (US9243038, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209043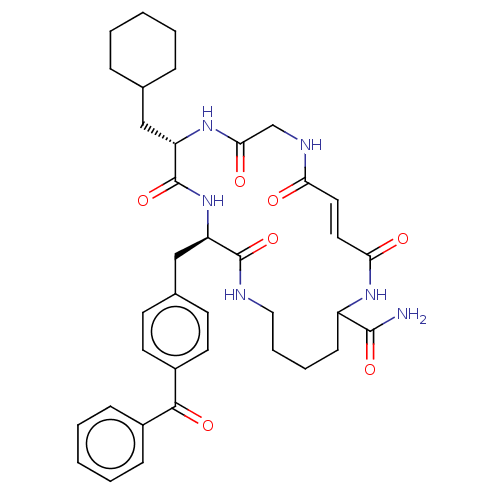 (US9243038, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209034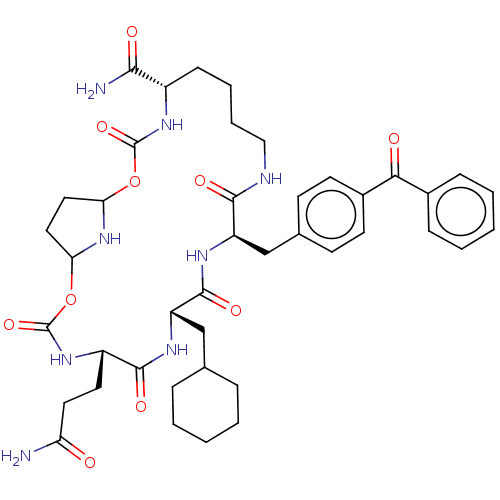 (US9243038, 6c ) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50738 (MLS000762832 | N-[(2,3-dimethoxyphenyl)methylidene...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 571 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005638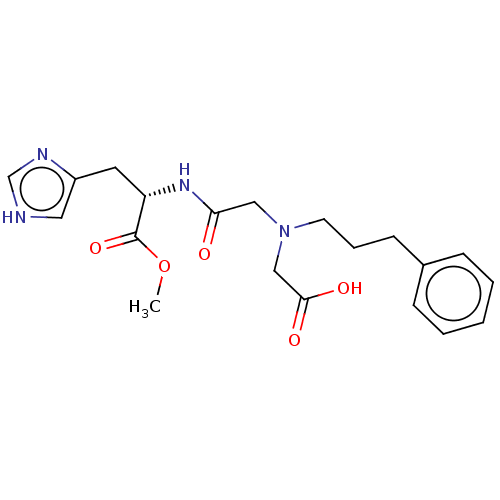 (CHEMBL3235414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005640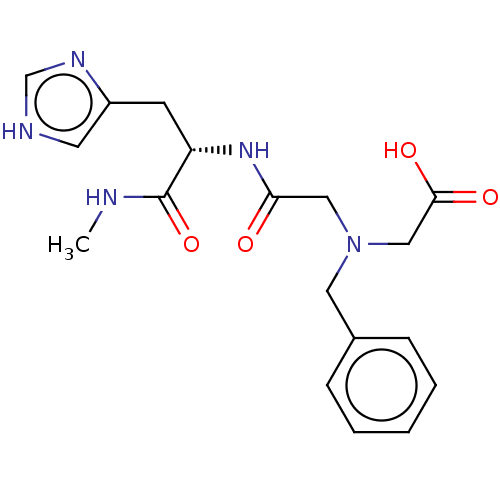 (CHEMBL3235415) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM314094 (US9610322, 5b (trans olefin)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description In vitro protease assays: Protease assays with IDE and neprilysin were performed using Mca-RPPGFSAFK(Dnp)-OH substrate peptide (R&D Systems, SEQ ID N... | US Patent US9610322 (2017) BindingDB Entry DOI: 10.7270/Q29Z970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209025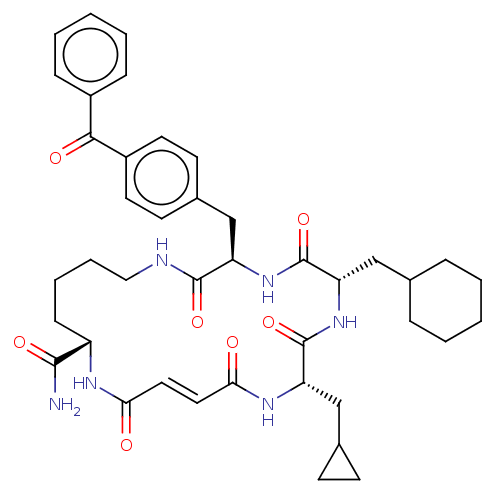 (US9243038, 5a (cis olefin) | US9243038, 5b (trans ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005637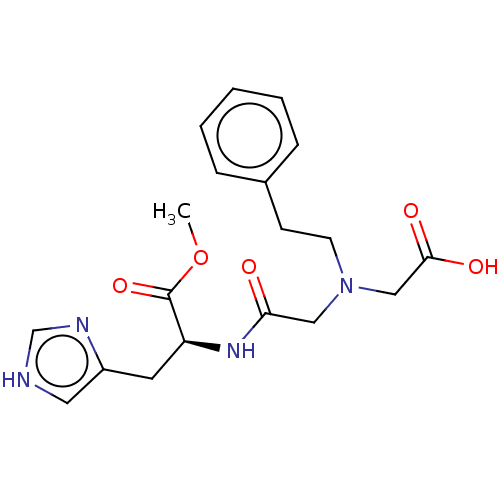 (CHEMBL3235413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM34336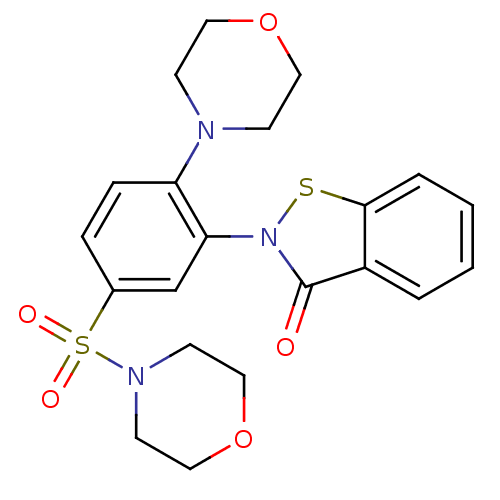 (2-(2-morpholin-4-yl-5-morpholin-4-ylsulfonyl-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM314089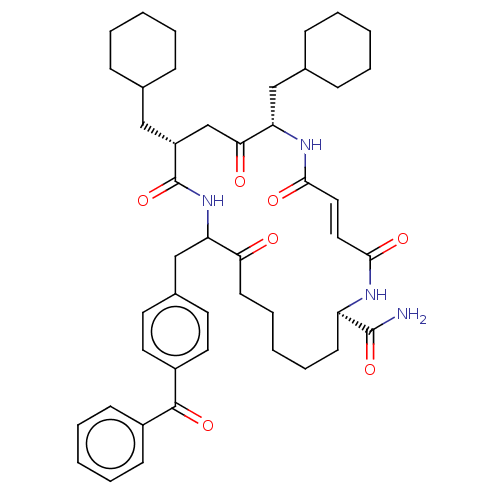 (US9610322, 3b (trans olefin)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description In vitro protease assays: Protease assays with IDE and neprilysin were performed using Mca-RPPGFSAFK(Dnp)-OH substrate peptide (R&D Systems, SEQ ID N... | US Patent US9610322 (2017) BindingDB Entry DOI: 10.7270/Q29Z970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209027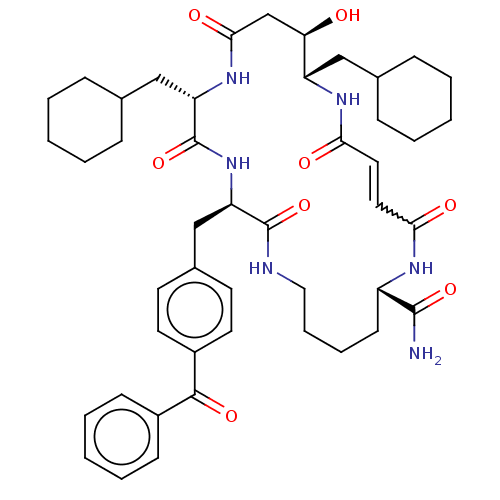 (US9243038, 3b (trans olefin)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209037 (US9243038, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM61086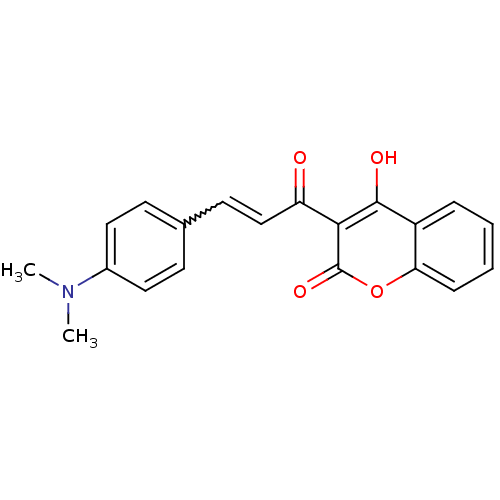 (3-[(E)-3-[4-(dimethylamino)phenyl]-1-oxoprop-2-eny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | >2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM75786 (2-(3-furanylmethylidene)-7-methyl-3-oxo-5-phenyl-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209038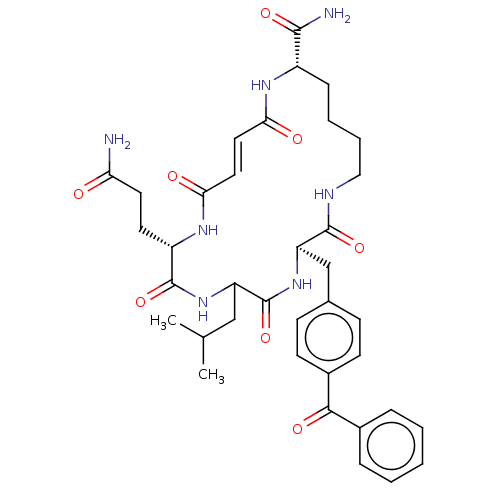 (US9243038, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM46036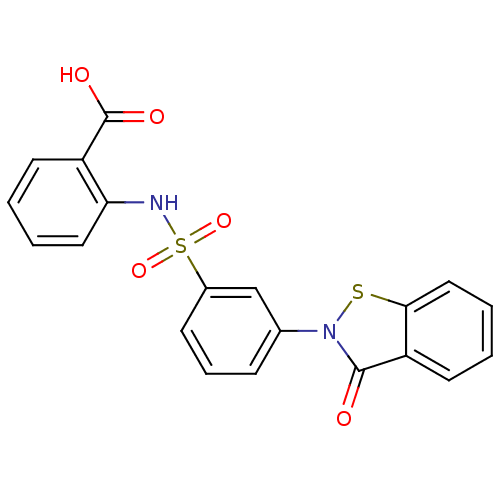 (2-[[3-(3-keto-1,2-benzothiazol-2-yl)phenyl]sulfony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM46058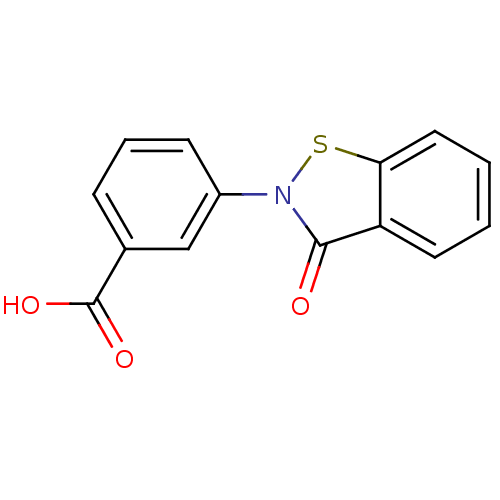 (3-(3-keto-1,2-benzothiazol-2-yl)benzoic acid | 3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM75784 (2-[[5-(3-chlorophenyl)-4-(furan-2-ylmethyl)-1,2,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM75800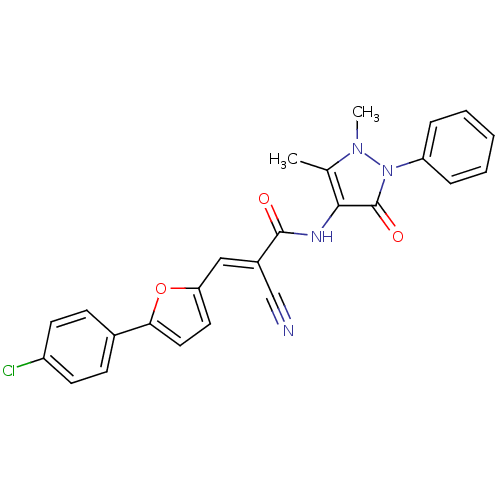 ((E)-3-[5-(4-chlorophenyl)-2-furanyl]-2-cyano-N-(1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005636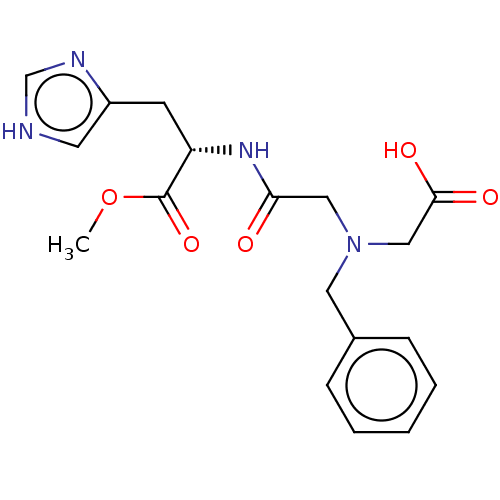 (CHEMBL3235412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM75787 (3-[[4-(2-methyl-2,3-dihydroindole-1-carbonyl)pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50005641 (CHEMBL3235416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE-mediated amyloid beta (1 to 40) hydrolysis preincubated for 10 mins measured after 30 mins by spectrophotometer a... | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209044 (US9243038, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM34981 (2,3-bis(oxidanyl)-1H-quinolin-4-one | 2,3-dihydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM75792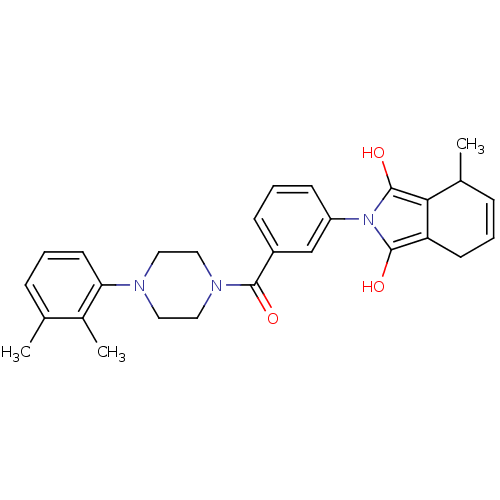 (2-[3-[4-(2,3-dimethylphenyl)piperazin-1-yl]carbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2416VHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 200 total ) | Next | Last >> |