

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for inhibition of skolin-stimulated adenylate cyclase in cell line expressing human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for inhibition of skolin-stimulated adenylate cyclase in cell line expressing human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130447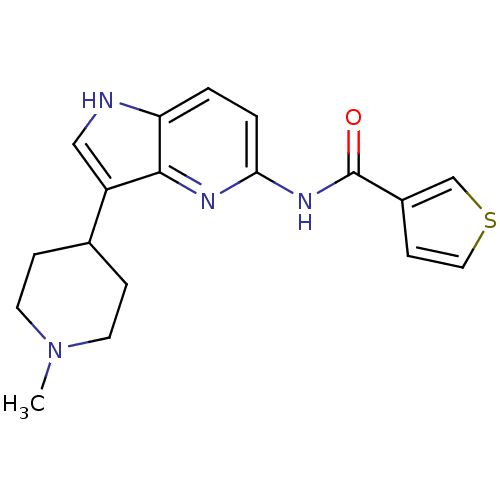 (CHEMBL104753 | Thiophene-3-carboxylic acid [3-(1-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130437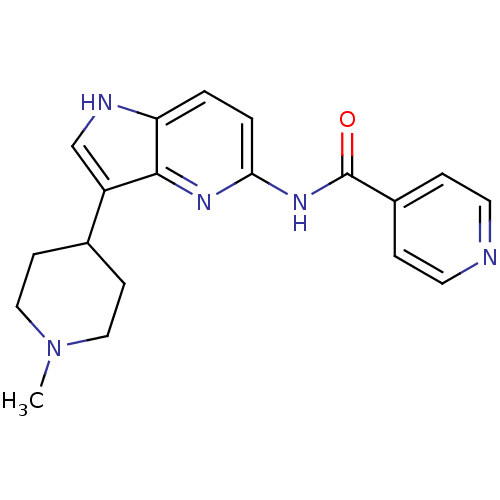 (CHEMBL105958 | N-[3-(1-Methyl-piperidin-4-yl)-1H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130420 (CHEMBL105091 | Furan-2-carboxylic acid [3-(1-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130439 (CHEMBL102250 | Furan-3-carboxylic acid [3-(1-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130465 (CHEMBL105955 | N-[3-(1-Methyl-piperidin-4-yl)-1H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130467 (CHEMBL321080 | Thiophene-2-carboxylic acid [3-(1-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130433 (CHEMBL420475 | Cyclobutanecarboxylic acid [3-(1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130442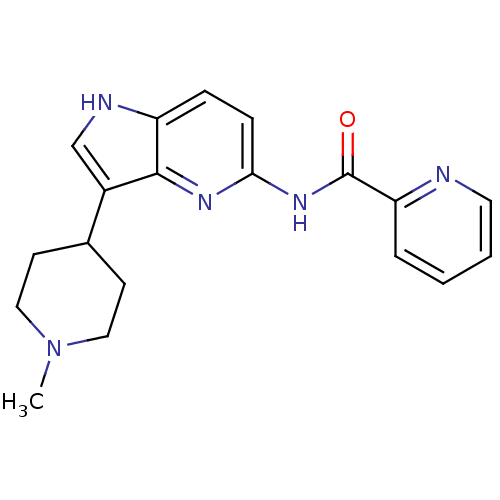 (CHEMBL105722 | Pyridine-2-carboxylic acid [3-(1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130426 (CHEMBL105261 | N-[3-(1-Methyl-piperidin-4-yl)-1H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130440 (CHEMBL104720 | Cyclopropanecarboxylic acid [3-(1-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-hydroxytryptamine 1F recep... | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for inhibition of skolin-stimulated adenylate cyclase in cell line expressing human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50130448 (CHEMBL431041 | N-[3-(1-Methyl-piperidin-4-yl)-1H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro effective concentration for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-)cells expressing the human 5-HT1F receptor | J Med Chem 46: 3060-71 (2003) Article DOI: 10.1021/jm030020m BindingDB Entry DOI: 10.7270/Q21V5DCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50156408 (2,4,6-Trifluoro-N-[3-(1-methyl-piperidin-4-yl)-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding to cloned human 5-hydroxytryptamine 1F receptor expressed in Mouse LM(tk-) cells | Bioorg Med Chem Lett 14: 6011-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.079 BindingDB Entry DOI: 10.7270/Q23J3CFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50119542 (CHEMBL3617550) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human 5HT1F receptor expressed in LM(tk-) cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 25: 4337-41 (2015) Article DOI: 10.1016/j.bmcl.2015.07.042 BindingDB Entry DOI: 10.7270/Q2NC630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50156403 (2-Chloro-4-fluoro-N-[3-(1-methyl-piperidin-4-yl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding to cloned human 5-hydroxytryptamine 1F receptor expressed in Mouse LM(tk-) cells | Bioorg Med Chem Lett 14: 6011-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.079 BindingDB Entry DOI: 10.7270/Q23J3CFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50137541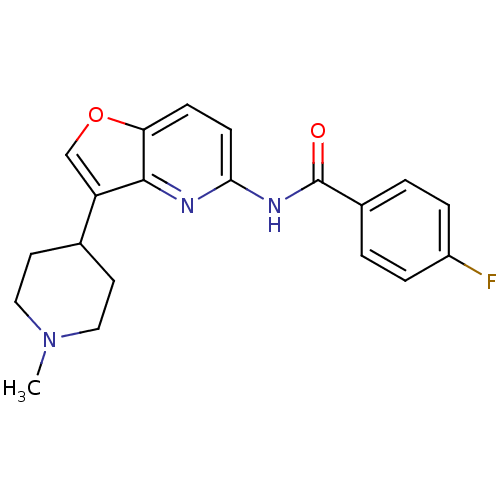 (4-Fluoro-N-[3-(1-methyl-piperidin-4-yl)-furo[3,2-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Concentration required for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-1) cells expressing the human 5-hydroxytryptamine 1F receptor | Bioorg Med Chem Lett 14: 167-70 (2003) BindingDB Entry DOI: 10.7270/Q2S46RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50137540 (2-Chloro-6-fluoro-N-[3-(1-methyl-piperidin-4-yl)-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Concentration required for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-1) cells expressing the human 5-hydroxytryptamine 1F receptor | Bioorg Med Chem Lett 14: 167-70 (2003) BindingDB Entry DOI: 10.7270/Q2S46RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50119543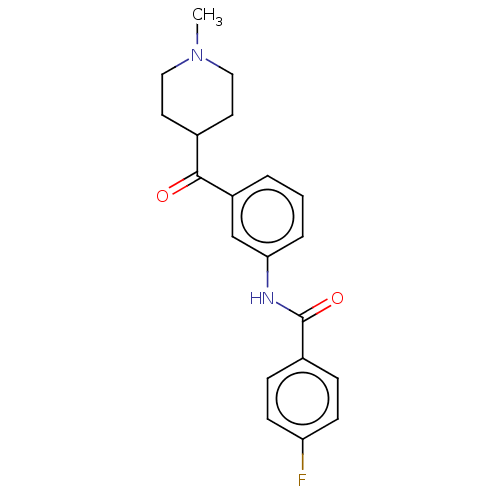 (CHEMBL3617549) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human 5HT1F receptor expressed in LM(tk-) cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 25: 4337-41 (2015) Article DOI: 10.1016/j.bmcl.2015.07.042 BindingDB Entry DOI: 10.7270/Q2NC630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50137539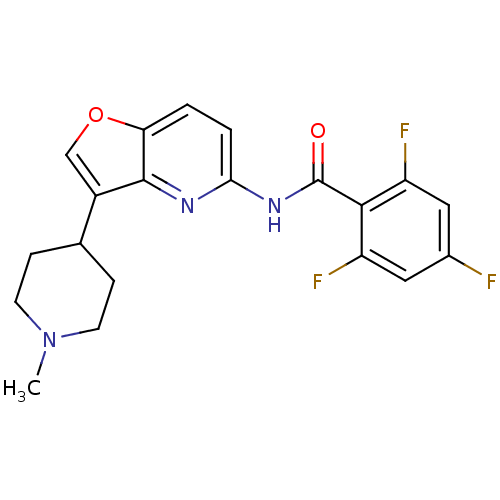 (2,4,6-Trifluoro-N-[3-(1-methyl-piperidin-4-yl)-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Concentration required for stimulation of [35S]-GTP-gammaS, binding in mouse LM(tk-1) cells expressing the human 5-hydroxytryptamine 1F receptor | Bioorg Med Chem Lett 14: 167-70 (2003) BindingDB Entry DOI: 10.7270/Q2S46RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50119532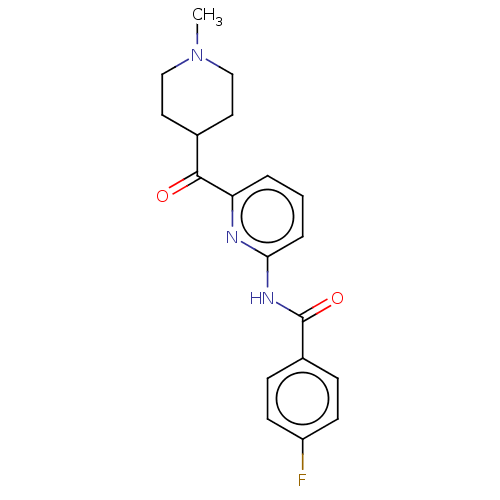 (CHEMBL3617557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human 5HT1F receptor expressed in LM(tk-) cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 25: 4337-41 (2015) Article DOI: 10.1016/j.bmcl.2015.07.042 BindingDB Entry DOI: 10.7270/Q2NC630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50156409 (2-Chloro-4-fluoro-N-[3-(1-methyl-piperidin-4-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding to cloned human 5-hydroxytryptamine 1F receptor expressed in Mouse LM(tk-) cells | Bioorg Med Chem Lett 14: 6011-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.079 BindingDB Entry DOI: 10.7270/Q23J3CFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50137538 (2-Chloro-4-fluoro-N-[3-(1-methyl-piperidin-4-yl)-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 277 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for human 5-hydroxytryptamine 1F receptor | Bioorg Med Chem Lett 14: 167-70 (2003) BindingDB Entry DOI: 10.7270/Q2S46RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50156406 (2,4-Difluoro-N-[3-(1-methyl-piperidin-4-yl)-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding to cloned human 5-hydroxytryptamine 1F receptor expressed in Mouse LM(tk-) cells | Bioorg Med Chem Lett 14: 6011-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.079 BindingDB Entry DOI: 10.7270/Q23J3CFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||