

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50288285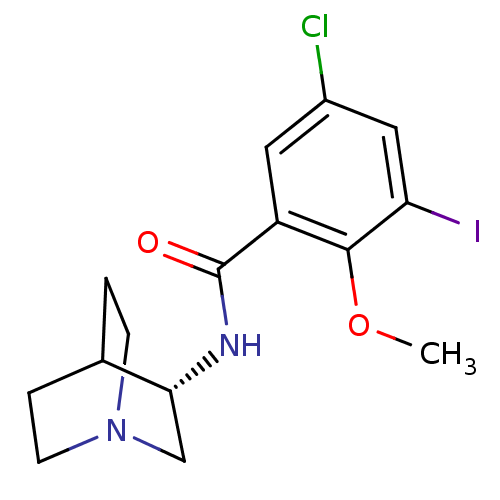 (CHEMBL90314 | N-(S)-1-Aza-bicyclo[2.2.2]oct-3-yl-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for its antagonistic activity against 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 6: 2657-2662 (1996) Article DOI: 10.1016/S0960-894X(96)00497-0 BindingDB Entry DOI: 10.7270/Q20V8CRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50417287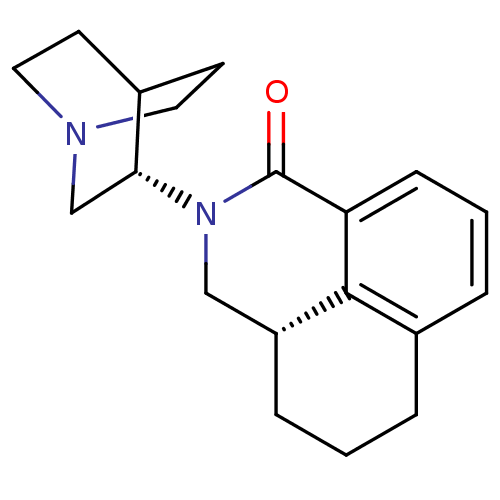 (Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Binding affinity to human wild type 5-HT3A receptor expressed in HEK293 cells after 24 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7523-8 (2013) Article DOI: 10.1016/j.bmc.2013.09.028 BindingDB Entry DOI: 10.7270/Q2JM2C3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50443668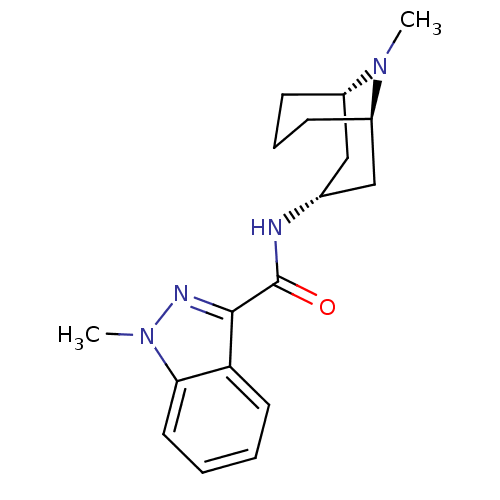 (CHEBI:5537 | CHEMBL1290003 | GRANISETRON) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Binding affinity to human wild type 5-HT3A receptor expressed in HEK293 cells after 24 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7523-8 (2013) Article DOI: 10.1016/j.bmc.2013.09.028 BindingDB Entry DOI: 10.7270/Q2JM2C3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its antagonistic activity against 5-hydroxytryptamine 3 receptor in rat brain using [3H]-zacopride as the radioligand. | Bioorg Med Chem Lett 6: 2657-2662 (1996) Article DOI: 10.1016/S0960-894X(96)00497-0 BindingDB Entry DOI: 10.7270/Q20V8CRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (RAT) | BDBM50288284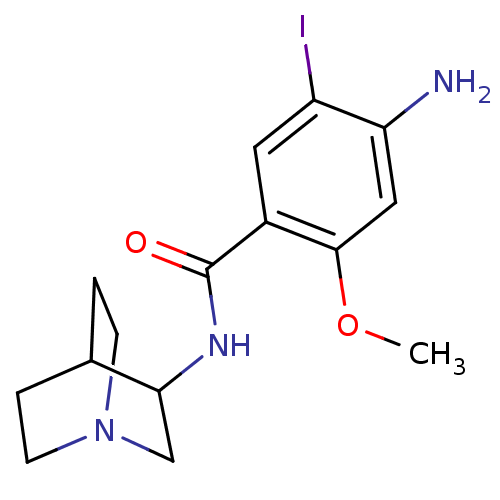 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-iodo-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards 5-HT-3 receptor in whole rat brain using (S)-[125I]-zacopride as the radioligand. | Bioorg Med Chem Lett 6: 2657-2662 (1996) Article DOI: 10.1016/S0960-894X(96)00497-0 BindingDB Entry DOI: 10.7270/Q20V8CRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (GUINEA PIG) | BDBM50060685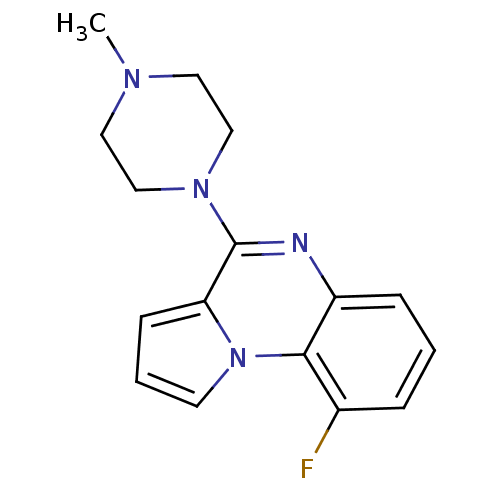 (9-Fluoro-4-(4-methyl-piperazin-1-yl)-pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development Curated by ChEMBL | Assay Description Antagonist activity at 5HT3 receptor in spontaneously beating guinea pig right atrium assessed as inhibition of serotonin-induced maximum response by... | J Med Chem 52: 6946-50 (2009) Article DOI: 10.1021/jm901126m BindingDB Entry DOI: 10.7270/Q2H9963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50426730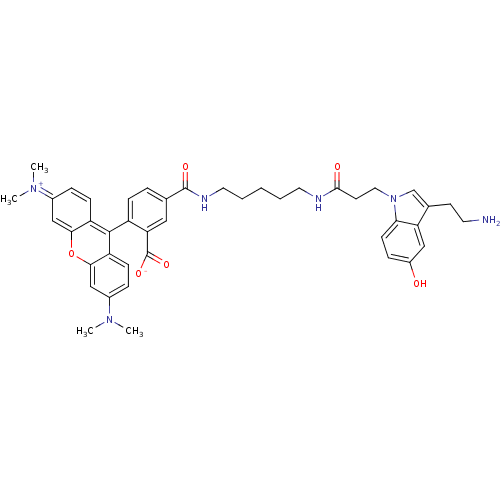 (CHEMBL2322133) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Binding affinity to C-terminal-His6-tagged 5HT3A receptor (unknown origin) expressed in HEK293 cells after 2 hrs by fluorimetric analysis | Bioorg Med Chem Lett 23: 773-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.082 BindingDB Entry DOI: 10.7270/Q2JD4Z35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (GUINEA PIG) | BDBM50081969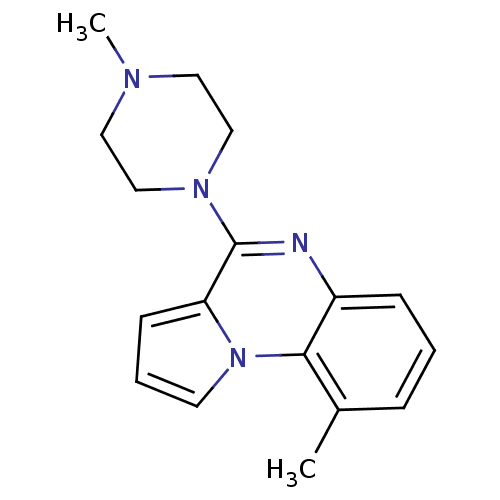 (9-Methyl-4-(4-methyl-piperazin-1-yl)-pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 955 | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development Curated by ChEMBL | Assay Description Antagonist activity at 5HT3 receptor in spontaneously beating guinea pig right atrium assessed as inhibition of serotonin-induced maximum response by... | J Med Chem 52: 6946-50 (2009) Article DOI: 10.1021/jm901126m BindingDB Entry DOI: 10.7270/Q2H9963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50248045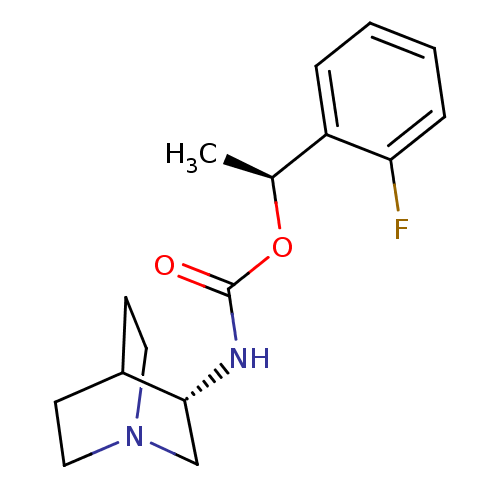 ((S)-1-(2-fluorophenyl)ethyl (S)-quinuclidin-3-ylca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc Curated by ChEMBL | Assay Description Binding affinity to 5HT3 receptor | J Med Chem 54: 7943-61 (2011) Article DOI: 10.1021/jm2007672 BindingDB Entry DOI: 10.7270/Q29P32QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||