 Found 1310 hits of ki data for polymerid = 49000083,49000084,49000086,49000087,49000089,8334
Found 1310 hits of ki data for polymerid = 49000083,49000084,49000086,49000087,49000089,8334 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
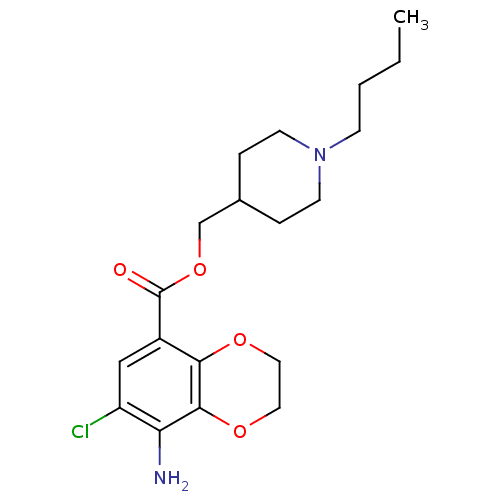
(RAT) | BDBM85027
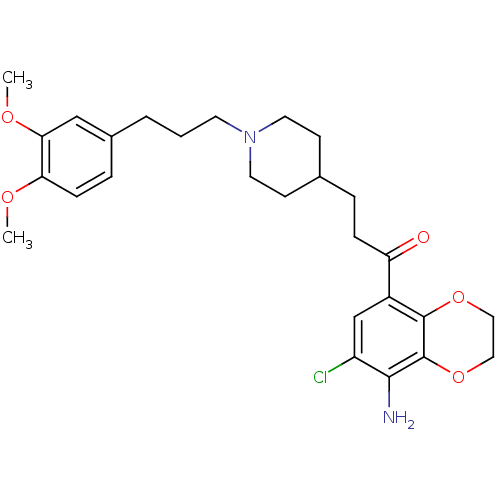
(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020
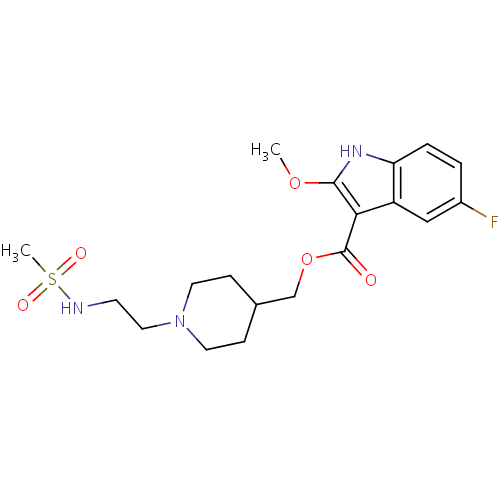
(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50483188
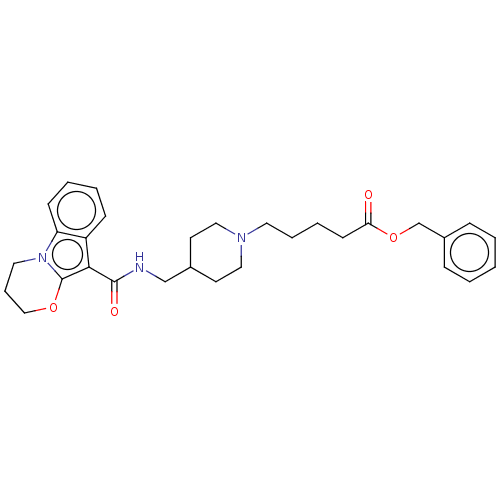
(CHEMBL1632166)Show SMILES O=C(CCCCN1CCC(CNC(=O)c2c3OCCCn3c3ccccc23)CC1)OCc1ccccc1 Show InChI InChI=1S/C30H37N3O4/c34-27(37-22-24-9-2-1-3-10-24)13-6-7-16-32-18-14-23(15-19-32)21-31-29(35)28-25-11-4-5-12-26(25)33-17-8-20-36-30(28)33/h1-5,9-12,23H,6-8,13-22H2,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 18: 8600-13 (2010)
Article DOI: 10.1016/j.bmc.2010.10.011
BindingDB Entry DOI: 10.7270/Q2FR00GH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85026
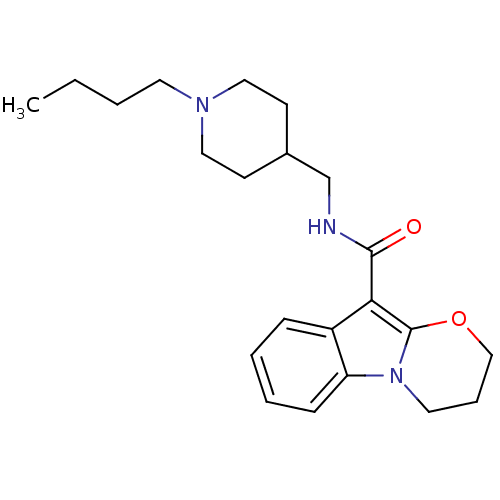
(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.00890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82505
(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.0129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for binding affinity against 5-HT4 receptor in guinea pig striata using [3H]GR-113808 as radioligand |
Citation and Details
Article DOI: 10.1016/S0960-894X(01)80414-5
BindingDB Entry DOI: 10.7270/Q2X63QND |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525
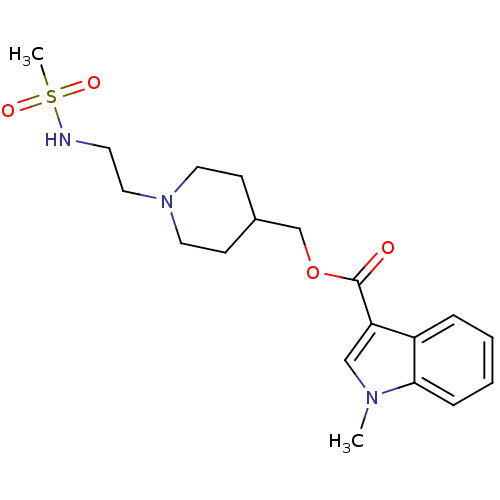
(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Eur J Pharmacol 298: 165-74 (1996)
Article DOI: 10.1016/0014-2999(95)00786-5
BindingDB Entry DOI: 10.7270/Q2GF0S1D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 74: 478-89 (2000)
Article DOI: 10.1046/j.1471-4159.2000.740478.x
BindingDB Entry DOI: 10.7270/Q2DJ5D5Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399614
(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis |
Eur J Med Chem 64: 629-37 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.060
BindingDB Entry DOI: 10.7270/Q27947NT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399614

(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50483175
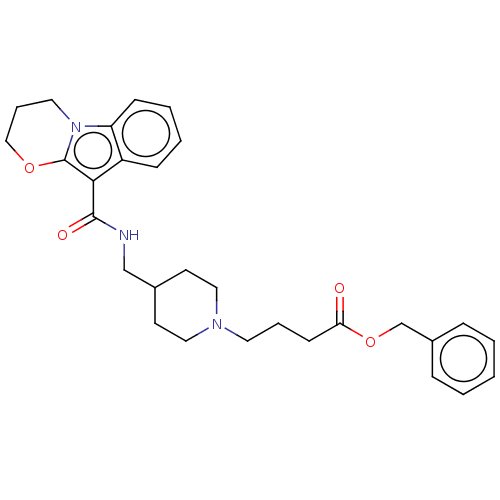
(CHEMBL1632165)Show SMILES O=C(CCCN1CCC(CNC(=O)c2c3OCCCn3c3ccccc23)CC1)OCc1ccccc1 Show InChI InChI=1S/C29H35N3O4/c33-26(36-21-23-8-2-1-3-9-23)12-6-15-31-17-13-22(14-18-31)20-30-28(34)27-24-10-4-5-11-25(24)32-16-7-19-35-29(27)32/h1-5,8-11,22H,6-7,12-21H2,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 18: 8600-13 (2010)
Article DOI: 10.1016/j.bmc.2010.10.011
BindingDB Entry DOI: 10.7270/Q2FR00GH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50327858
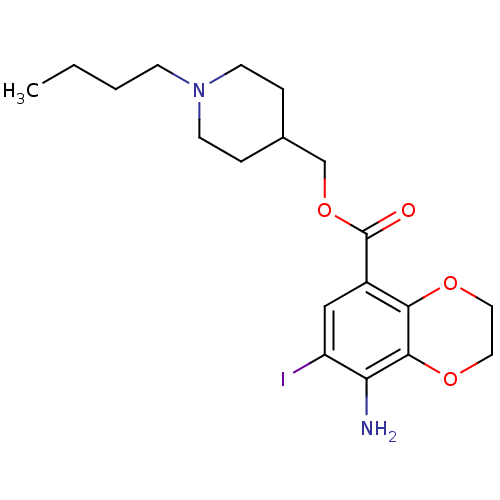
((1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-d...)Show InChI InChI=1S/C19H27IN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
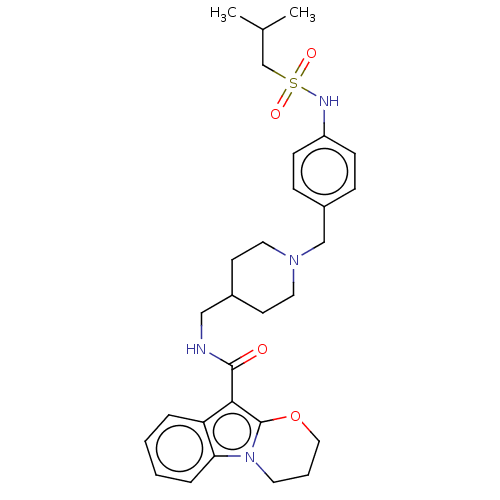
(Homo sapiens (Human)) | BDBM50023377
(CHEMBL3329805)Show SMILES CC(C)CS(=O)(=O)Nc1ccc(CN2CCC(CNC(=O)c3c4OCCCn4c4ccccc34)CC2)cc1 Show InChI InChI=1S/C29H38N4O4S/c1-21(2)20-38(35,36)31-24-10-8-23(9-11-24)19-32-15-12-22(13-16-32)18-30-28(34)27-25-6-3-4-7-26(25)33-14-5-17-37-29(27)33/h3-4,6-11,21-22,31H,5,12-20H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4b receptor expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 4598-602 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.083
BindingDB Entry DOI: 10.7270/Q28W3FW7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 18: 8600-13 (2010)
Article DOI: 10.1016/j.bmc.2010.10.011
BindingDB Entry DOI: 10.7270/Q2FR00GH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
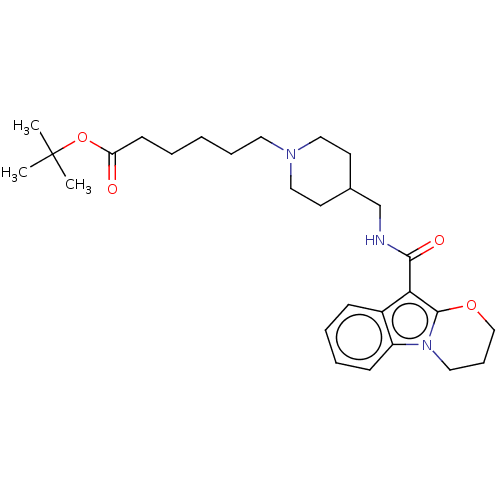
(Homo sapiens (Human)) | BDBM50483174
(CHEMBL1632163)Show SMILES CC(C)(C)OC(=O)CCCCCN1CCC(CNC(=O)c2c3OCCCn3c3ccccc23)CC1 Show InChI InChI=1S/C28H41N3O4/c1-28(2,3)35-24(32)12-5-4-8-15-30-17-13-21(14-18-30)20-29-26(33)25-22-10-6-7-11-23(22)31-16-9-19-34-27(25)31/h6-7,10-11,21H,4-5,8-9,12-20H2,1-3H3,(H,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 18: 8600-13 (2010)
Article DOI: 10.1016/j.bmc.2010.10.011
BindingDB Entry DOI: 10.7270/Q2FR00GH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 74: 478-89 (2000)
Article DOI: 10.1046/j.1471-4159.2000.740478.x
BindingDB Entry DOI: 10.7270/Q2DJ5D5Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
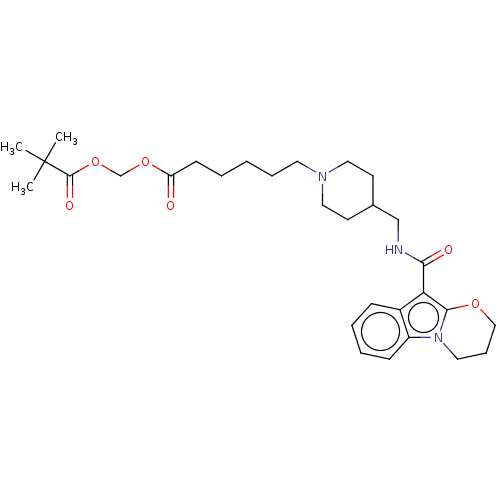
(Homo sapiens (Human)) | BDBM50483187
(CHEMBL1632169)Show SMILES CC(C)(C)C(=O)OCOC(=O)CCCCCN1CCC(CNC(=O)c2c3OCCCn3c3ccccc23)CC1 Show InChI InChI=1S/C30H43N3O6/c1-30(2,3)29(36)39-21-38-25(34)12-5-4-8-15-32-17-13-22(14-18-32)20-31-27(35)26-23-10-6-7-11-24(23)33-16-9-19-37-28(26)33/h6-7,10-11,22H,4-5,8-9,12-21H2,1-3H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4 receptor expressed in HEK293 cells by liquid scintillation counting |
Bioorg Med Chem 18: 8600-13 (2010)
Article DOI: 10.1016/j.bmc.2010.10.011
BindingDB Entry DOI: 10.7270/Q2FR00GH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by PDSP Ki Database
| |
J Neurochem 70: 2252-61 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70062252.x
BindingDB Entry DOI: 10.7270/Q2Z036PW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 69: 1810-9 (1997)
Article DOI: 10.1046/j.1471-4159.1997.69051810.x
BindingDB Entry DOI: 10.7270/Q2Z60MMW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Antagonistic activity at human 5HT4 receptor expressed in HEK293 cells |
Bioorg Med Chem 18: 8600-13 (2010)
Article DOI: 10.1016/j.bmc.2010.10.011
BindingDB Entry DOI: 10.7270/Q2FR00GH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity was determined against cloned 5-hydroxytryptamine 4D receptor isoform expressed in COS-7 cells |
J Med Chem 43: 3761-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CC0ZXH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50327858

((1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-d...)Show InChI InChI=1S/C19H27IN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398985
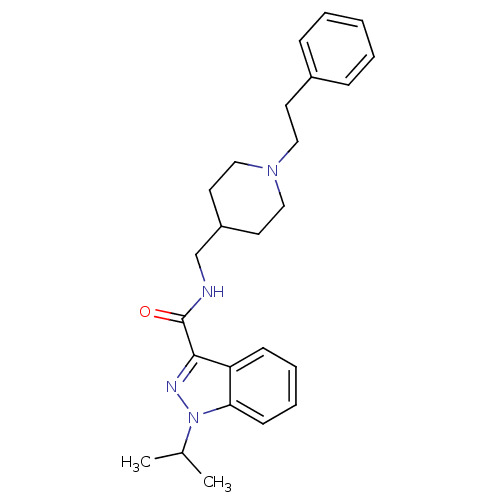
(CHEMBL2177130)Show SMILES CC(C)n1nc(C(=O)NCC2CCN(CCc3ccccc3)CC2)c2ccccc12 Show InChI InChI=1S/C25H32N4O/c1-19(2)29-23-11-7-6-10-22(23)24(27-29)25(30)26-18-21-13-16-28(17-14-21)15-12-20-8-4-3-5-9-20/h3-11,19,21H,12-18H2,1-2H3,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells |
J Med Chem 55: 9446-66 (2012)
Article DOI: 10.1021/jm300573d
BindingDB Entry DOI: 10.7270/Q27082JT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50023365
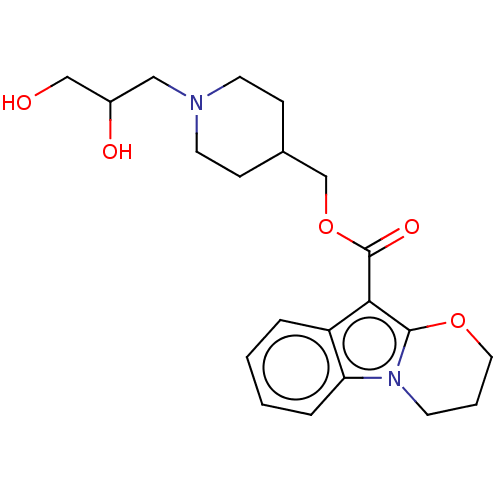
(CHEMBL3329814)Show SMILES OCC(O)CN1CCC(COC(=O)c2c3OCCCn3c3ccccc23)CC1 Show InChI InChI=1S/C21H28N2O5/c24-13-16(25)12-22-9-6-15(7-10-22)14-28-21(26)19-17-4-1-2-5-18(17)23-8-3-11-27-20(19)23/h1-2,4-5,15-16,24-25H,3,6-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4b receptor expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 4598-602 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.083
BindingDB Entry DOI: 10.7270/Q28W3FW7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398983
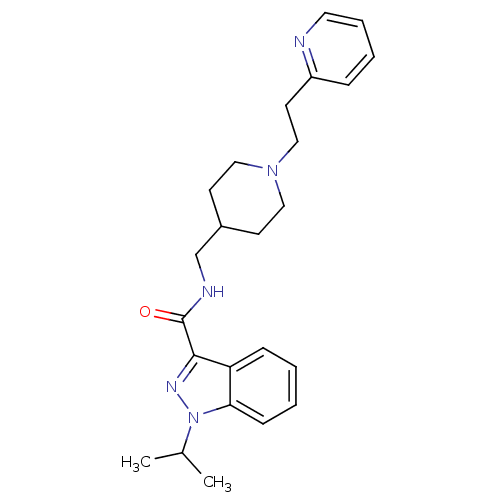
(CHEMBL2179697)Show SMILES CC(C)n1nc(C(=O)NCC2CCN(CCc3ccccn3)CC2)c2ccccc12 Show InChI InChI=1S/C24H31N5O/c1-18(2)29-22-9-4-3-8-21(22)23(27-29)24(30)26-17-19-10-14-28(15-11-19)16-12-20-7-5-6-13-25-20/h3-9,13,18-19H,10-12,14-17H2,1-2H3,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center
Curated by ChEMBL
| Assay Description
Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells |
J Med Chem 55: 9446-66 (2012)
Article DOI: 10.1021/jm300573d
BindingDB Entry DOI: 10.7270/Q27082JT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was evaluated for binding affinity against 5-hydroxytryptamine 4 receptor using radioligand binding assay using [3H]GR as radioligand |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2CTV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis |
Eur J Med Chem 64: 629-37 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.060
BindingDB Entry DOI: 10.7270/Q27947NT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 69: 1810-9 (1997)
Article DOI: 10.1046/j.1471-4159.1997.69051810.x
BindingDB Entry DOI: 10.7270/Q2Z60MMW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Neurochem 74: 478-89 (2000)
Article DOI: 10.1046/j.1471-4159.2000.740478.x
BindingDB Entry DOI: 10.7270/Q2DJ5D5Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity was determined against cloned 5-hydroxytryptamine 4E receptor isoform expressed in C6 glial cells incubated with 0.2 nM [3H]-GR-113,... |
J Med Chem 43: 3761-9 (2000)
BindingDB Entry DOI: 10.7270/Q2CC0ZXH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
FASEB J 10: 1398-407 (1996)
Article DOI: 10.1096/fasebj.10.12.8903510
BindingDB Entry DOI: 10.7270/Q2TQ602G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data