

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50268077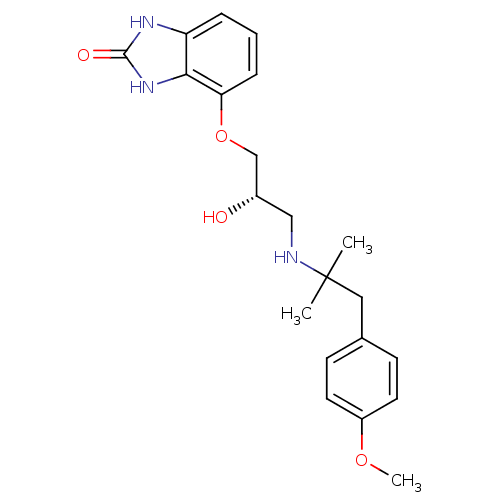 ((S)-4-(2-hydroxy-3-(1-(4-methoxyphenyl)-2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of beta2 adrenergic receptor | J Med Chem 52: 6599-605 (2009) Article DOI: 10.1021/jm900563e BindingDB Entry DOI: 10.7270/Q2WM1FB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50407517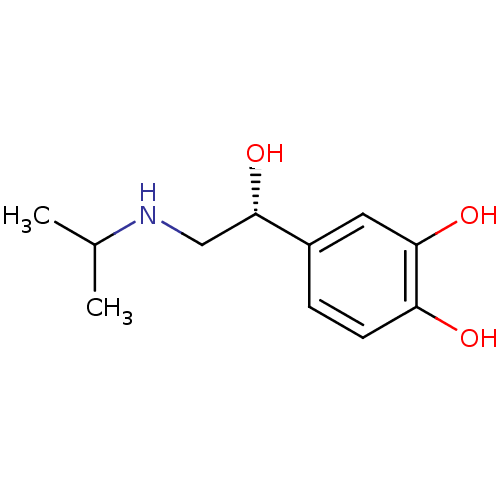 ((-)ISOPROTERENOL | CHEMBL1160723 | US9492405, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Rattus norvegicus) | BDBM25392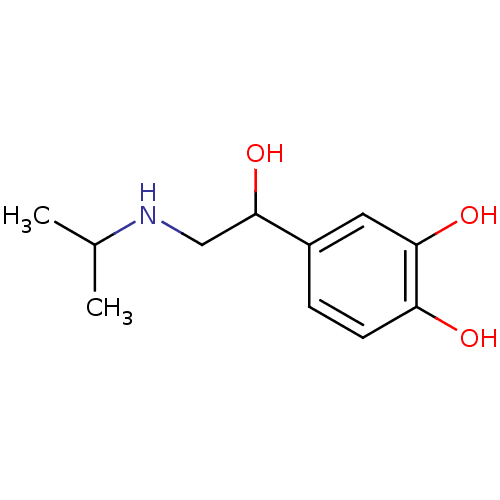 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of spontaneous contractions in isolated rat uterus | J Med Chem 46: 105-12 (2002) Article DOI: 10.1021/jm020177z BindingDB Entry DOI: 10.7270/Q2BP03J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213098 ((R,R)-(-)-fenoterol | CHEMBL388570 | US10617654, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213099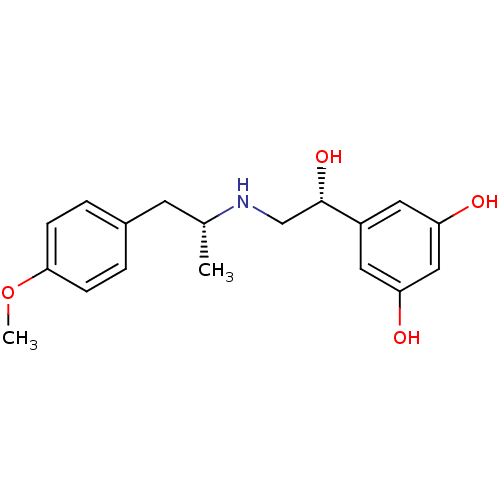 ((R,R)-(-)-1-p-methoxyphenyl-2-(beta-3',5'-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419668 (CHEMBL1947157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419678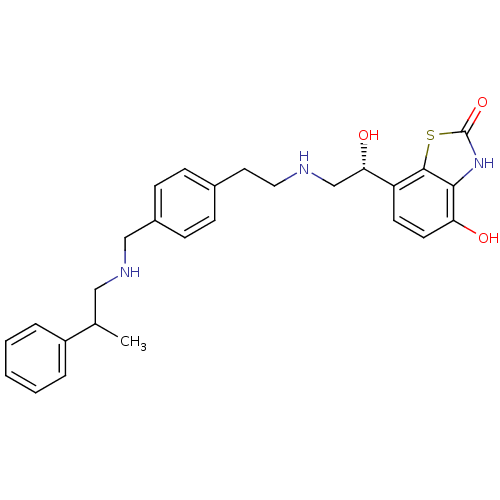 (CHEMBL1945036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221910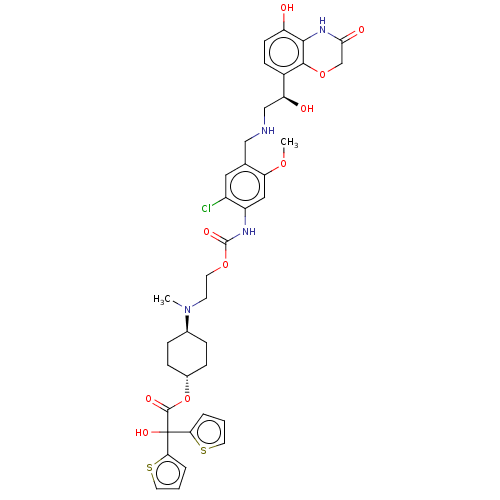 (US9315463, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25761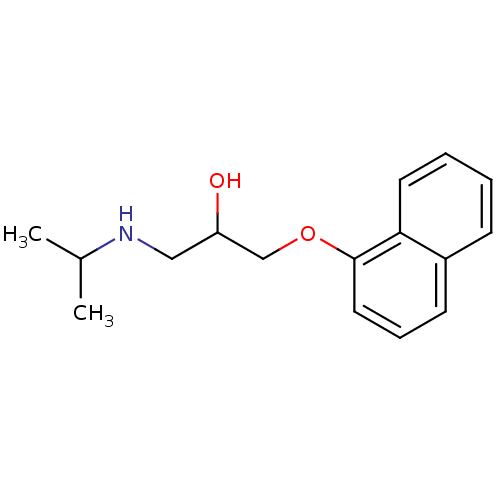 (Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Antagonist activity at ADRB2 endogenously expressed in HEK293 cells transfected with cAMP FRET biosensor assessed as inhibition of cimaterol-induced ... | J Med Chem 63: 8458-8470 (2020) Article DOI: 10.1021/acs.jmedchem.0c00831 BindingDB Entry DOI: 10.7270/Q25D8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419670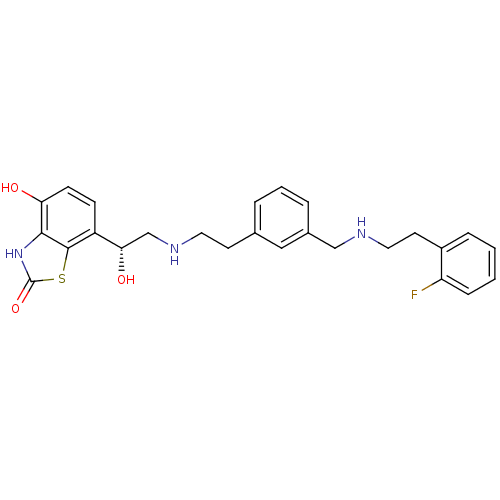 (CHEMBL1944691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419702 (CHEMBL1945038) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25761 (Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Antagonist activity at ADRB2 endogenously expressed in HEK293 cells transfected with cAMP FRET biosensor assessed as inhibition of cimaterol-induced ... | J Med Chem 63: 8458-8470 (2020) Article DOI: 10.1021/acs.jmedchem.0c00831 BindingDB Entry DOI: 10.7270/Q25D8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419710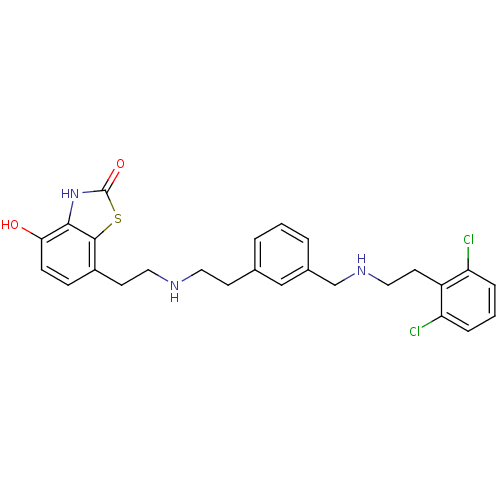 (CHEMBL1946768) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419669 (CHEMBL1944690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419674 (CHEMBL1945031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518977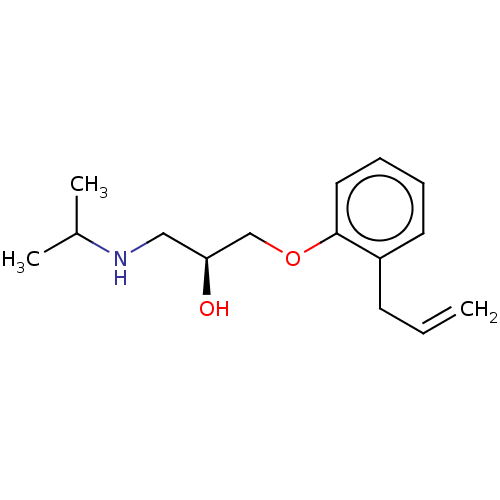 (CHEMBL1160734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25768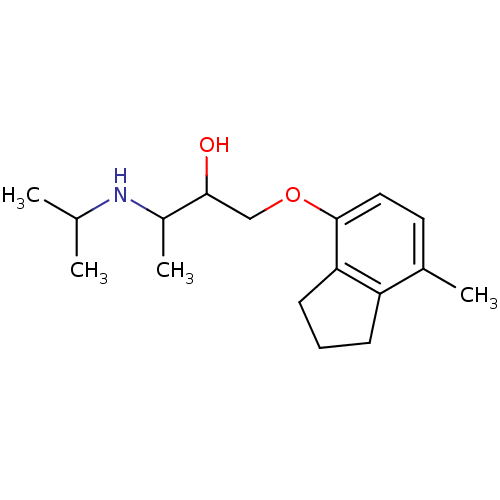 (1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H](-)CGP12177 from human recombinant Beta-2 adrenergic receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27960 ((2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]CGP 12177 from human recombinant beta2 adrenergic receptor expressed in CHO cells measured after 120 mins by scintillation counti... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419679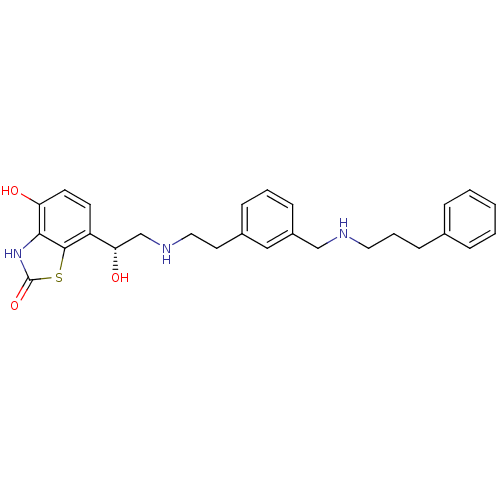 (CHEMBL1945037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419671 (CHEMBL1944693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419687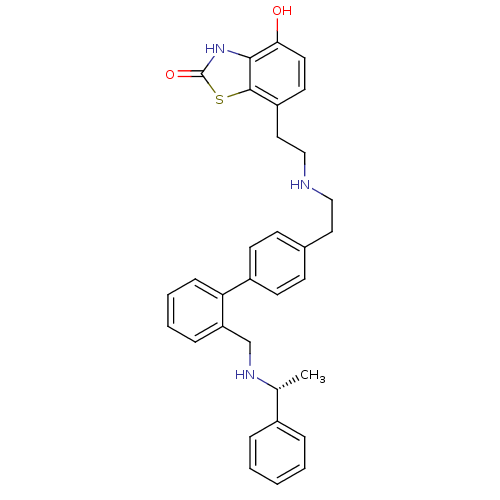 (CHEMBL1947151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50268076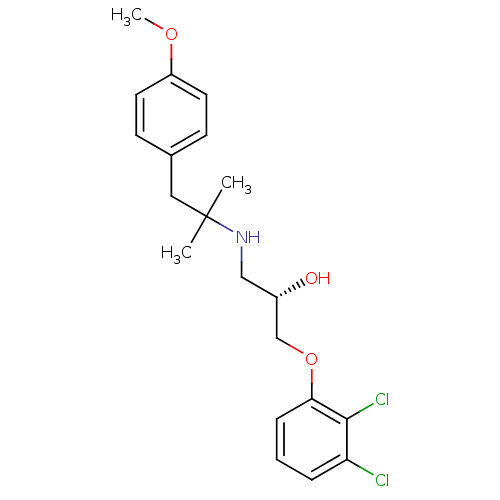 ((2S)-1-[(2,3-Dichlorophenyl)oxy]-3-((1,1-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to beta2 adrenoceptor | J Med Chem 52: 3982-93 (2009) Article DOI: 10.1021/jm900364m BindingDB Entry DOI: 10.7270/Q2M61K5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419703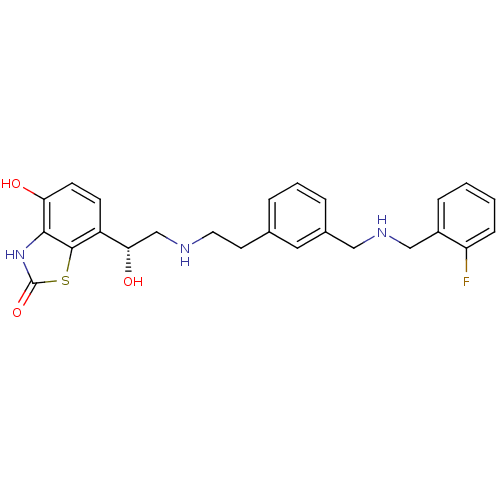 (CHEMBL1945042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50520335 (CHEMBL4465173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Medicines Curated by ChEMBL | Assay Description Inhibition of beta2 adrenoreceptor (unknown origin) | J Med Chem 63: 4430-4444 (2020) Article DOI: 10.1021/acs.jmedchem.9b01581 BindingDB Entry DOI: 10.7270/Q2ZG6WM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221912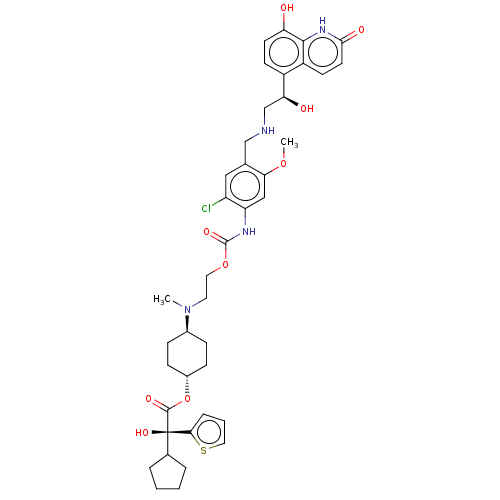 (US9315463, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25764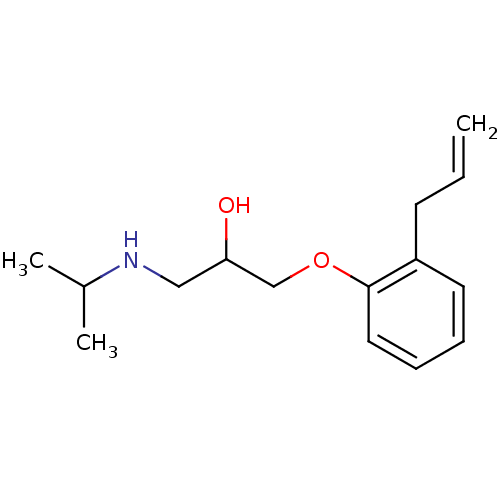 (ALPRENOLOL,(+) | ALPRENOLOL,(-) | Alfeprol | Alphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213110 ((R,S)-(-)-5-{1-hydroxy-2-[1-methyl-2-(1-naphthyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.19 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518969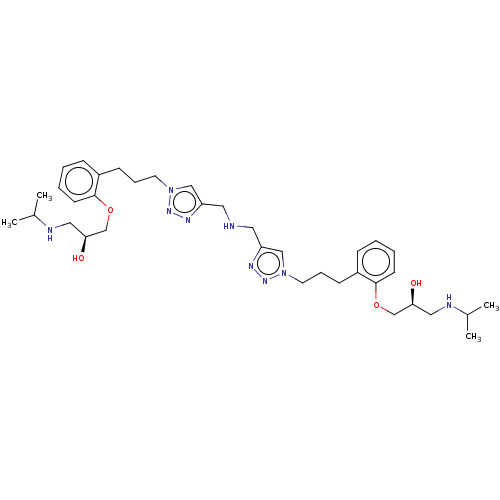 (CHEMBL4514723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221911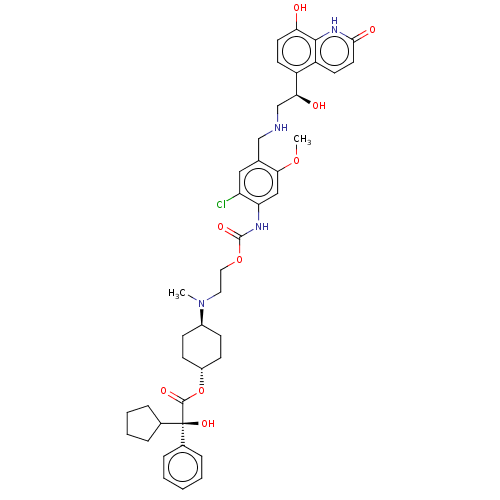 (US9315463, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50268077 ((S)-4-(2-hydroxy-3-(1-(4-methoxyphenyl)-2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to beta2 adrenoceptor | J Med Chem 52: 3982-93 (2009) Article DOI: 10.1021/jm900364m BindingDB Entry DOI: 10.7270/Q2M61K5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM200753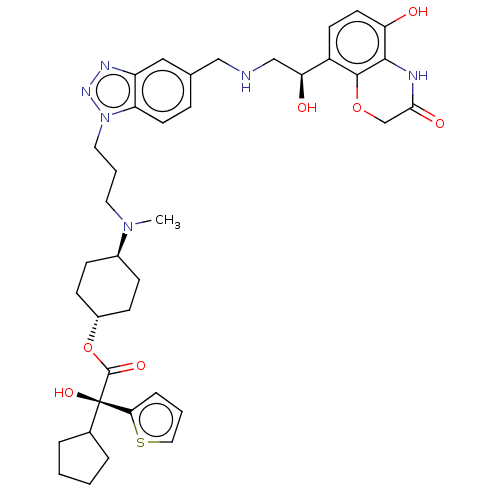 (US9233108, 18 | US9757383, Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9757383 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM200753 (US9233108, 18 | US9757383, Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | 25 |
ALMIRALL, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9233108 (2016) BindingDB Entry DOI: 10.7270/Q23J3BSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50348426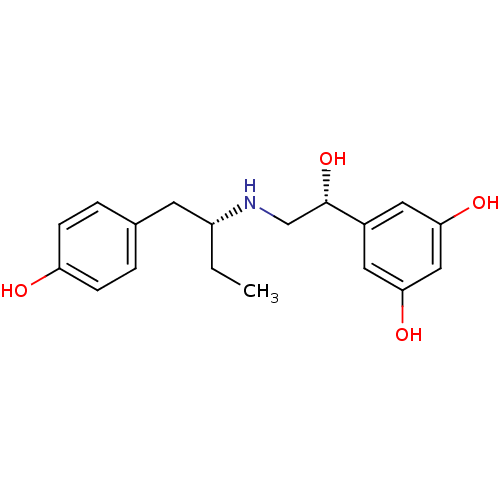 (CHEMBL1800936 | US9492405, 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM200750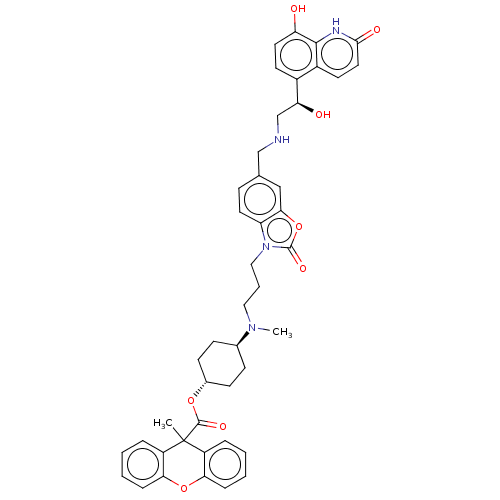 (US9233108, 15 | US9757383, Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ALMIRALL, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9233108 (2016) BindingDB Entry DOI: 10.7270/Q23J3BSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM200750 (US9233108, 15 | US9757383, Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9757383 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518981 (CHEMBL4594173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213115 ((R,R)-(-)-5-{1-hydroxy-2-[1-methyl-2-(1-naphthyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419665 (CHEMBL1945299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518978 (CHEMBL4446394) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221902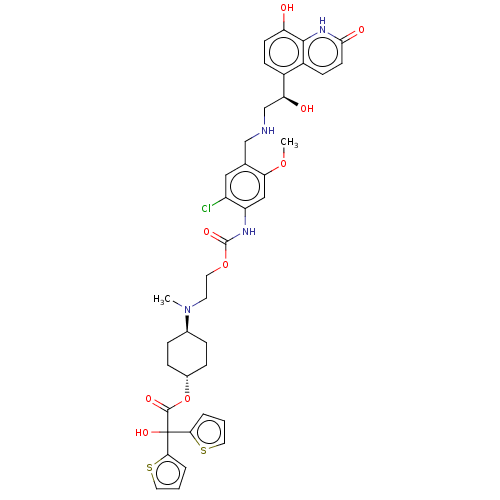 (US9315463, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518984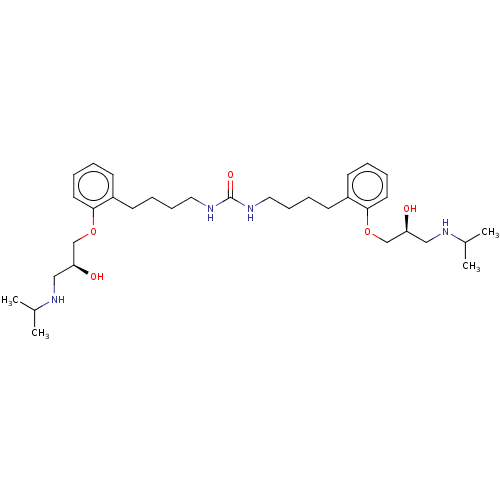 (CHEMBL4564189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) stably expressed in HEK293 cell membranes assessed as inhibition of isoproterenol-i... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50545798 (CHEMBL4633760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Antagonist activity at ADRB2 endogenously expressed in HEK293 cells transfected with cAMP FRET biosensor assessed as inhibition of cimaterol-induced ... | J Med Chem 63: 8458-8470 (2020) Article DOI: 10.1021/acs.jmedchem.0c00831 BindingDB Entry DOI: 10.7270/Q25D8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518985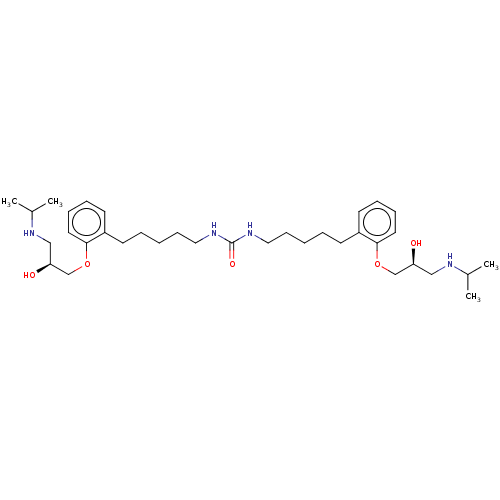 (CHEMBL4465278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) stably expressed in HEK293 cell membranes assessed as inhibition of isoproterenol-i... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518984 (CHEMBL4564189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50348428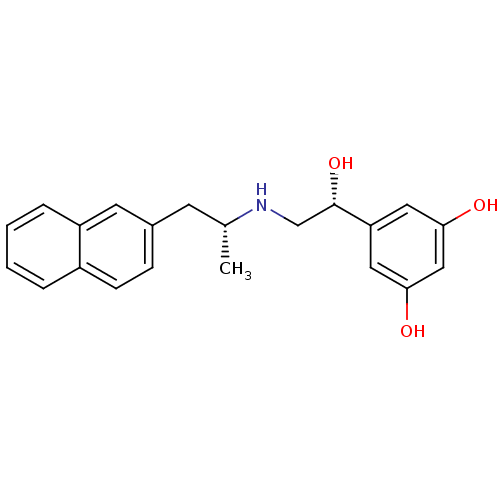 (CHEMBL1800961 | US9492405, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | 37 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description To measure beta2-AR mediated inhibition of mitogenesis, HEK-beta2-AR, 1321N1 or U87MG cells were seeded in a 96-well plate at approximately 5,000 c... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419673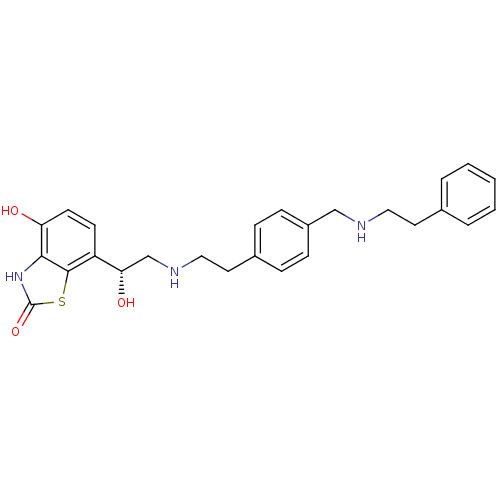 (CHEMBL1944695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419675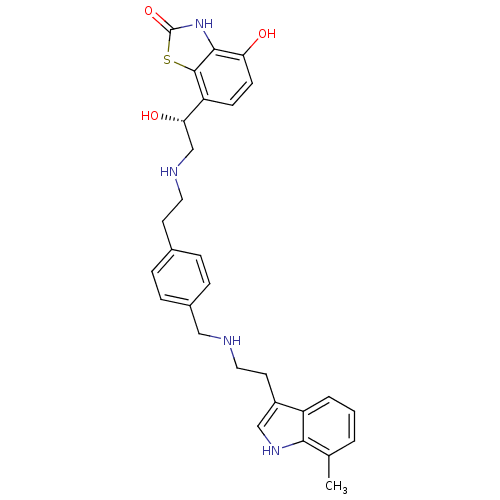 (CHEMBL1945032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419701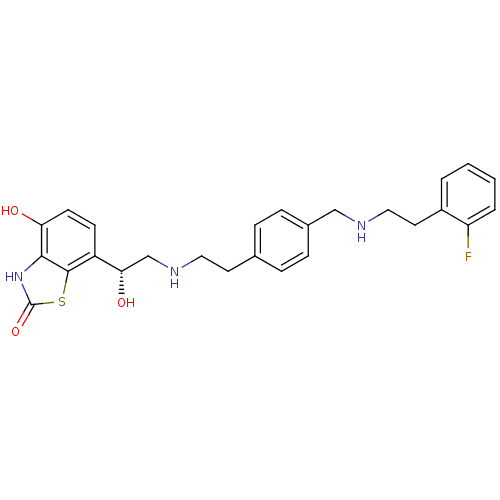 (CHEMBL1944692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50518982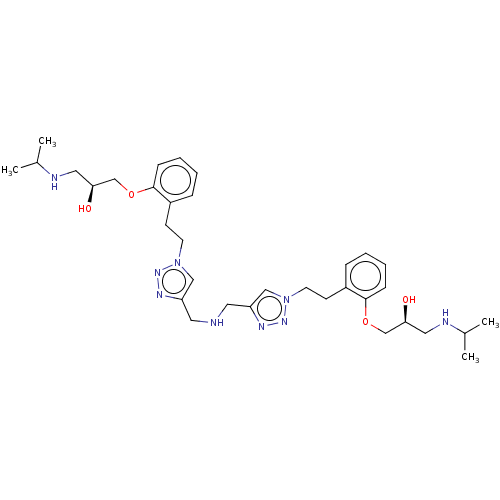 (CHEMBL4462252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at beta2 adrenergic receptor (unknown origin) expressed in HEK293 cell assessed as inhibition of isoproterenol-induced cAMP produ... | J Med Chem 62: 7806-7839 (2019) Article DOI: 10.1021/acs.jmedchem.9b00595 BindingDB Entry DOI: 10.7270/Q2PG1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50419698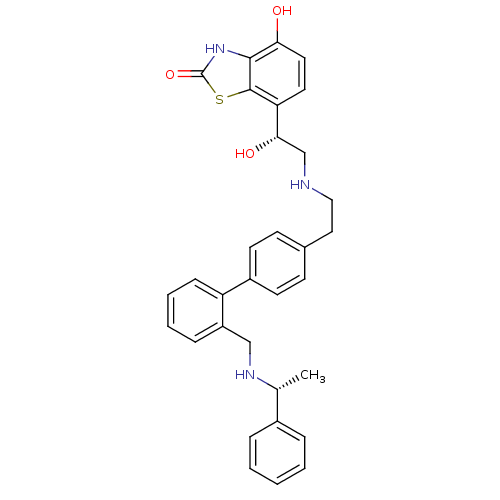 (CHEMBL1947155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of radiolabeled iodocyanopindolol from recombinant beta2 adrenoceptor | Bioorg Med Chem Lett 22: 689-95 (2011) Article DOI: 10.1016/j.bmcl.2011.10.049 BindingDB Entry DOI: 10.7270/Q2KW5H90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 719 total ) | Next | Last >> |