

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (RAT) | BDBM50409528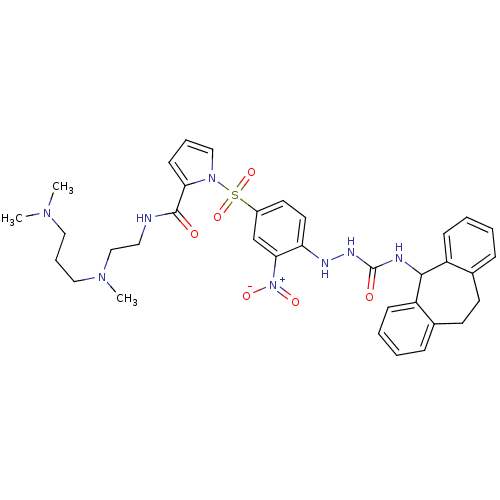 (CHEMBL2112220) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscle | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409527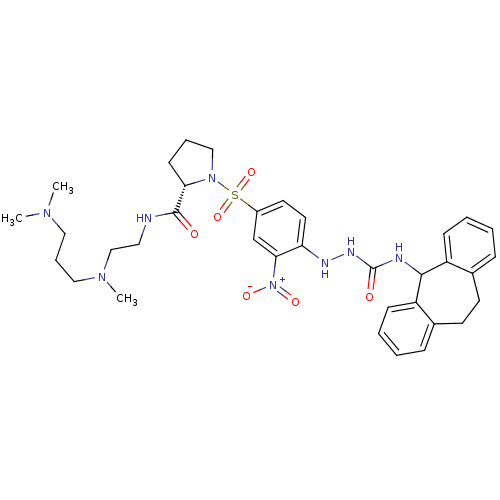 (CHEMBL2112283) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscle | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409527 (CHEMBL2112283) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2 | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409528 (CHEMBL2112220) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2 | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097740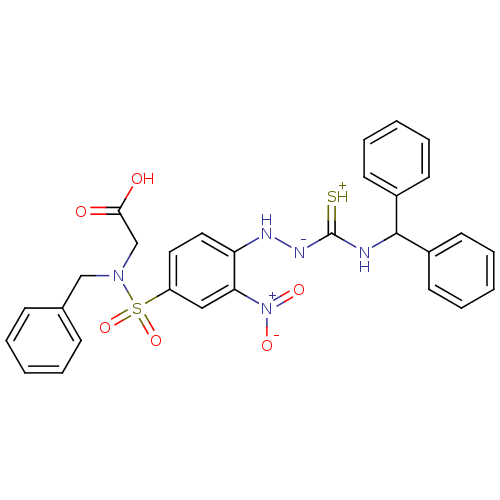 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097736 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097727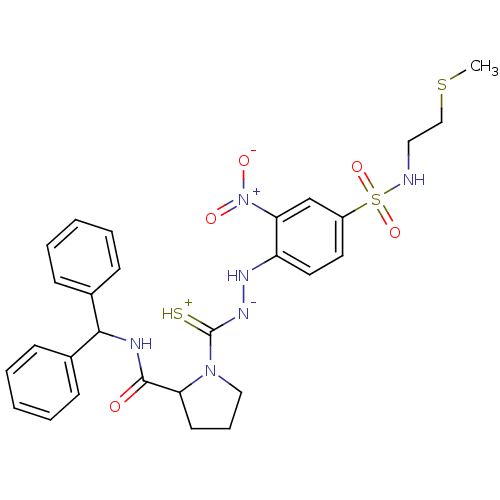 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097732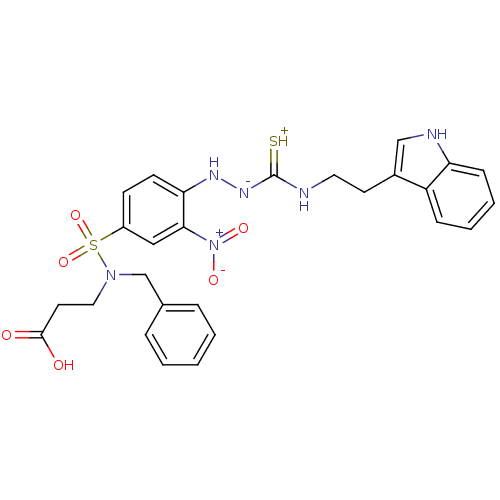 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097737 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097730 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097723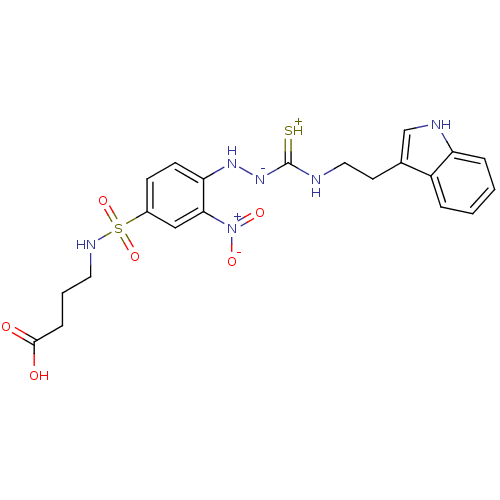 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097734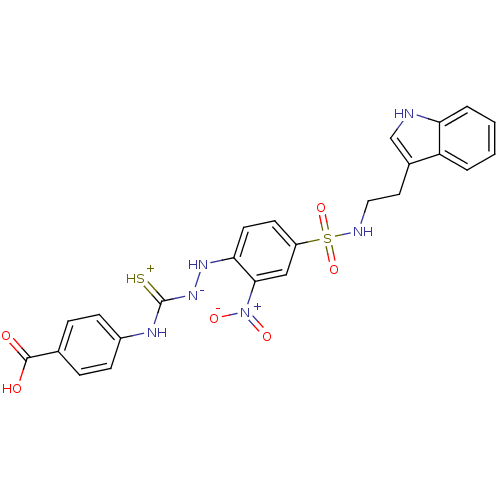 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097742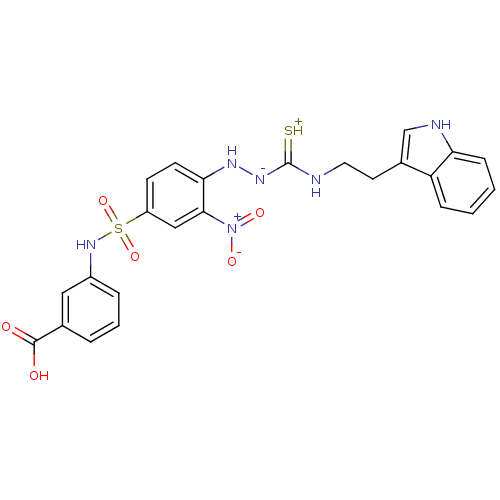 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097726 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097738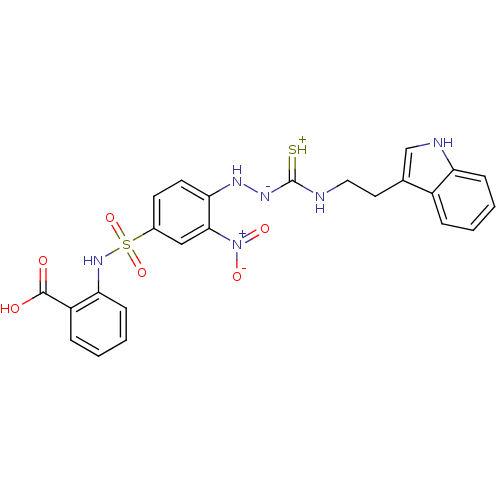 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097731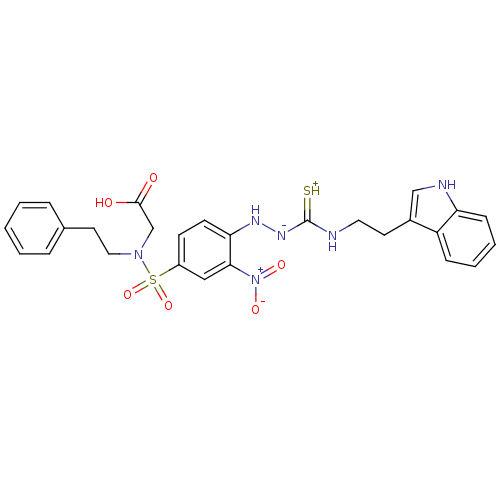 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097743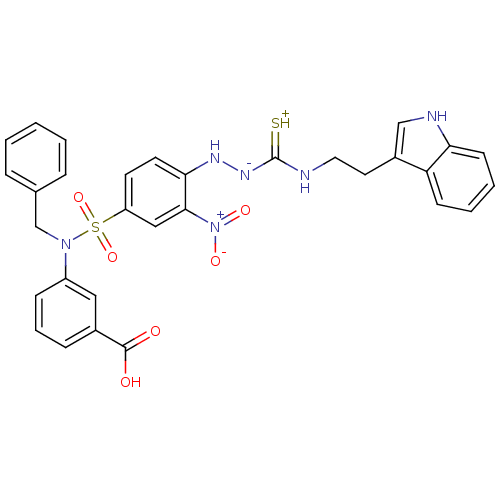 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097724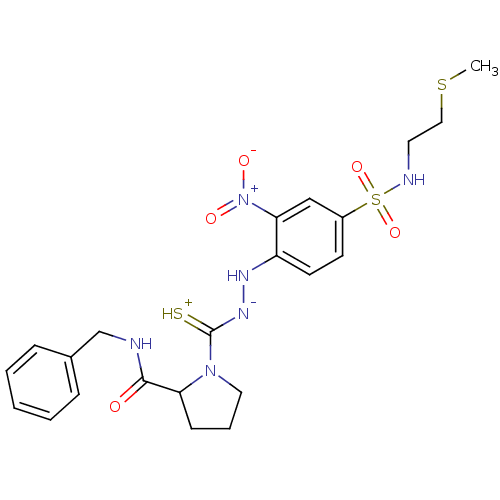 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097733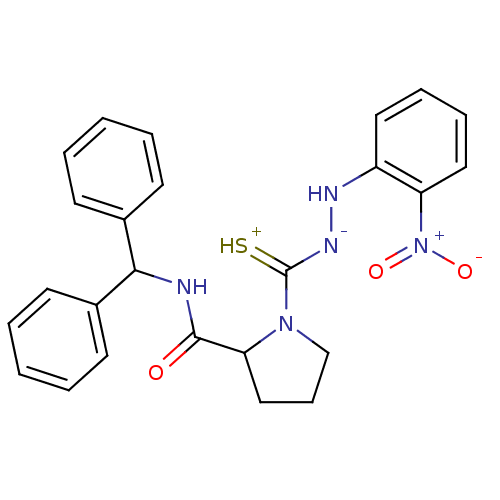 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097733 ((S)-1-[N'-(2-Nitro-phenyl)-hydrazinothiocarbonyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097728 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097729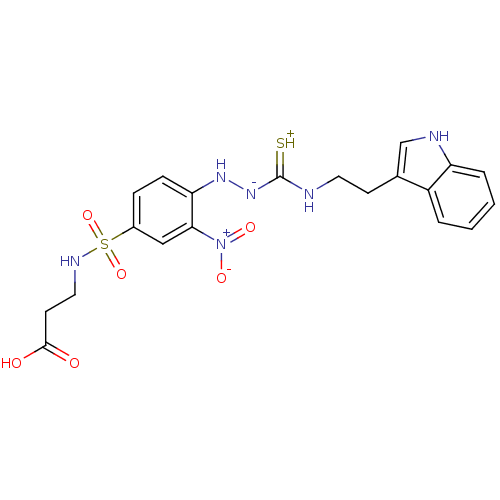 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097727 ((S)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097724 ((R)-1-{N'-[4-(2-Methylsulfanyl-ethylsulfamoyl)-2-n...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50097725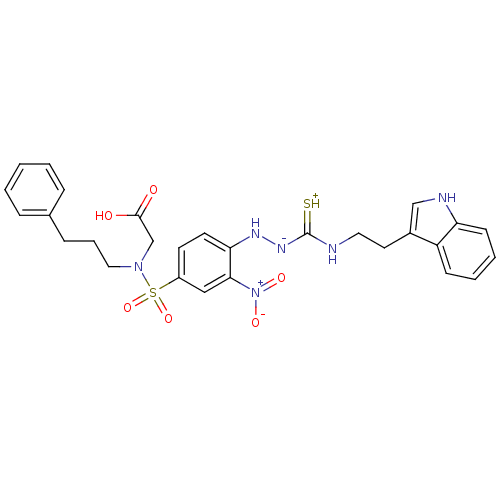 (1-(2-nitrophenyl)thiosemicarbazide analogue | CHEM...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay | Bioorg Med Chem Lett 11: 705-9 (2001) BindingDB Entry DOI: 10.7270/Q22V2GNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||