 Found 737 hits of ki for UniProtKB: P10980
Found 737 hits of ki for UniProtKB: P10980 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021919
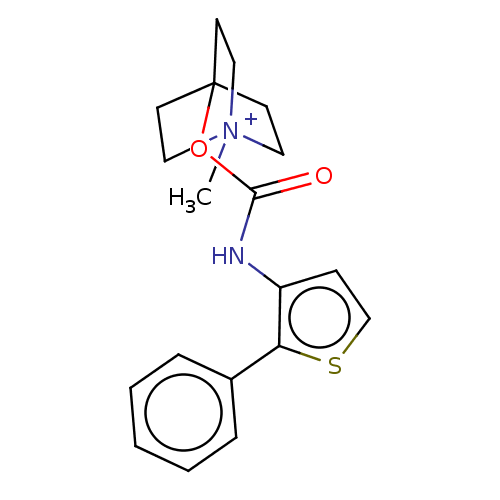
(CHEMBL3298595)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ccsc1-c1ccccc1 Show InChI InChI=1S/C19H22N2O2S.HI/c1-21-11-8-19(9-12-21,10-13-21)23-18(22)20-16-7-14-24-17(16)15-5-3-2-4-6-15;/h2-7,14H,8-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592
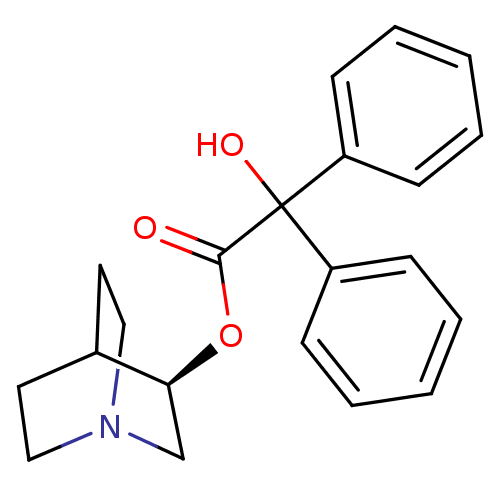
(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB (quinuclidinyl benzylate) from muscarinic M2 receptor of rat heart homogenates. |
Bioorg Med Chem Lett 7: 979-984 (1997)
Article DOI: 10.1016/S0960-894X(97)00143-1
BindingDB Entry DOI: 10.7270/Q2N29XFM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-quinuclidinyl benzilate(QNB) from muscarinic (M2) receptor in rat heart homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021928
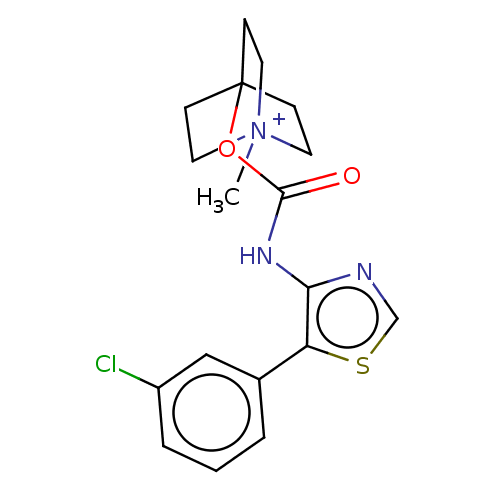
(CHEMBL3298599)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-8-5-18(6-9-22,7-10-22)24-17(23)21-16-15(25-12-20-16)13-3-2-4-14(19)11-13;/h2-4,11-12H,5-10H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121132
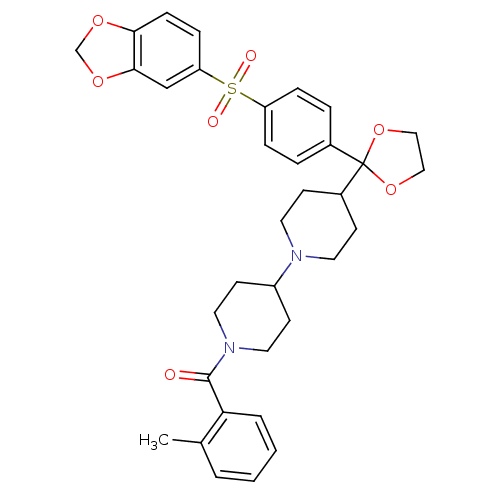
((4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1...)Show SMILES Cc1ccccc1C(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C34H38N2O7S/c1-24-4-2-3-5-30(24)33(37)36-18-14-27(15-19-36)35-16-12-26(13-17-35)34(42-20-21-43-34)25-6-8-28(9-7-25)44(38,39)29-10-11-31-32(22-29)41-23-40-31/h2-11,22,26-27H,12-21,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50055976
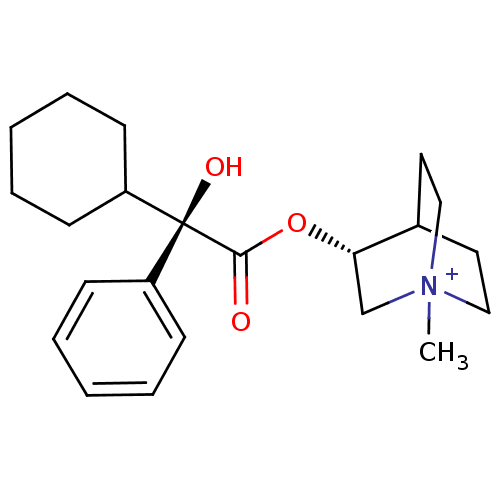
((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,wD:12.13,7.10,TLB:9:7:2.3:6.5,(13.94,-4.53,;13.94,-2.99,;14.71,-1.66,;13.24,-1.26,;14.01,.06,;15.34,-.72,;15.34,-2.26,;12.68,-.71,;12.68,-2.25,;11.35,.06,;10.02,-.71,;10.02,-2.26,;8.66,.09,;9.46,1.42,;7.33,.86,;6,.09,;4.67,.86,;4.67,2.4,;6,3.17,;7.33,2.4,;7.89,-1.27,;6.35,-1.27,;5.58,-2.6,;6.35,-3.94,;7.89,-3.95,;8.66,-2.62,)| Show InChI InChI=1S/C22H32NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2,4-5,8-9,17,19-20,25H,3,6-7,10-16H2,1H3/q+1/t17?,20-,22-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021922
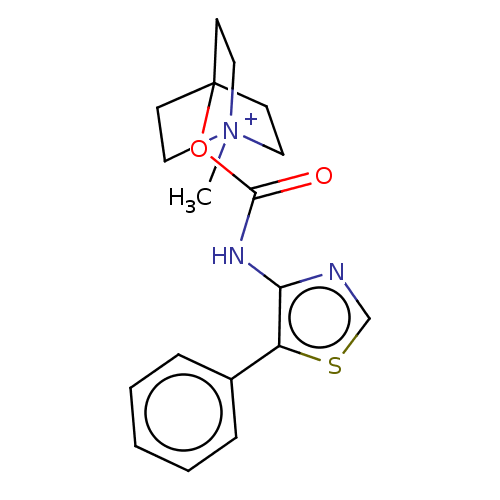
(CHEMBL3298596)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccccc1 Show InChI InChI=1S/C18H21N3O2S.HI/c1-21-10-7-18(8-11-21,9-12-21)23-17(22)20-16-15(24-13-19-16)14-5-3-2-4-6-14;/h2-6,13H,7-12H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132
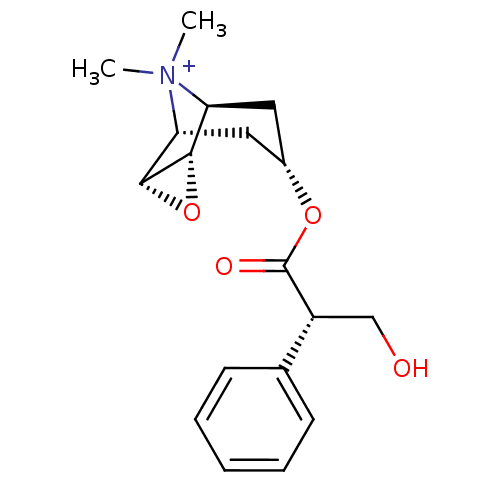
(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Binding affinity to Muscarinic acetylcholine receptor M2 by measuring its ability to displace [3H]N-methylscopolamine binding in rat heart |
J Med Chem 41: 3220-31 (1998)
Article DOI: 10.1021/jm9708588
BindingDB Entry DOI: 10.7270/Q2M32ZH9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50368152
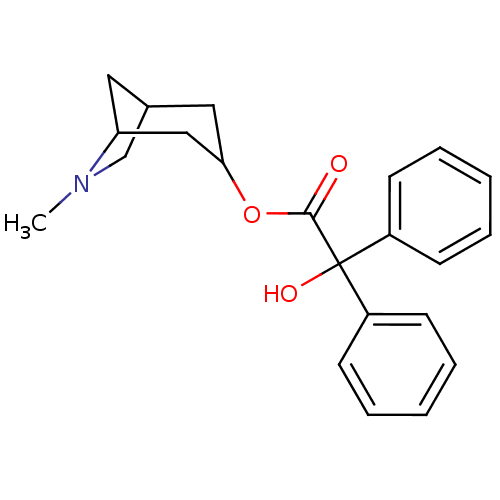
(CHEMBL318812)Show SMILES CN1CC2CC1CC(C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |THB:9:7:1.2:4,0:1:4:6.7.8| Show InChI InChI=1S/C22H25NO3/c1-23-15-16-12-19(23)14-20(13-16)26-21(24)22(25,17-8-4-2-5-9-17)18-10-6-3-7-11-18/h2-11,16,19-20,25H,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Compound was tested for binding activity against rat muscarinic acetylcholine receptor M2 using [3H]QNB as the radioligand |
J Med Chem 34: 3164-71 (1991)
BindingDB Entry DOI: 10.7270/Q2V988Q7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50010096
(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 |
J Med Chem 38: 1711-9 (1995)
BindingDB Entry DOI: 10.7270/Q2PV6JDQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021935
(CHEMBL3298600)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1ccc(F)cc1 Show InChI InChI=1S/C18H20FN3O2S.HI/c1-22-9-6-18(7-10-22,8-11-22)24-17(23)21-16-15(25-12-20-16)13-2-4-14(19)5-3-13;/h2-5,12H,6-11H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50055978
(4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...)Show SMILES C[N+]1(C)CCC(CC1)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NO3/c1-22(2)15-13-19(14-16-22)25-20(23)21(24,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3,5-6,9-10,18-19,24H,4,7-8,11-16H2,1-2H3/q+1/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50010096

(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM82559
(methoctramine analog 5)Show SMILES CN(CCCCCCNCCCCCCCCNCCCCCCN(CN1c2ccccc2C(=O)Nc2cccnc12)CN1c2ccccc2C(=O)Nc2cccnc12)CN1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C60H75N13O3/c1-69(43-71-52-31-13-10-25-46(52)58(74)66-49-28-22-38-63-55(49)71)41-20-8-6-18-36-61-34-16-4-2-3-5-17-35-62-37-19-7-9-21-42-70(44-72-53-32-14-11-26-47(53)59(75)67-50-29-23-39-64-56(50)72)45-73-54-33-15-12-27-48(54)60(76)68-51-30-24-40-65-57(51)73/h10-15,22-33,38-40,61-62H,2-9,16-21,34-37,41-45H2,1H3,(H,66,74)(H,67,75)(H,68,76) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by PDSP Ki Database
| |
J Med Chem 36: 3734-7 (1993)
Article DOI: 10.1021/jm00075a032
BindingDB Entry DOI: 10.7270/Q2W957Q0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM82423
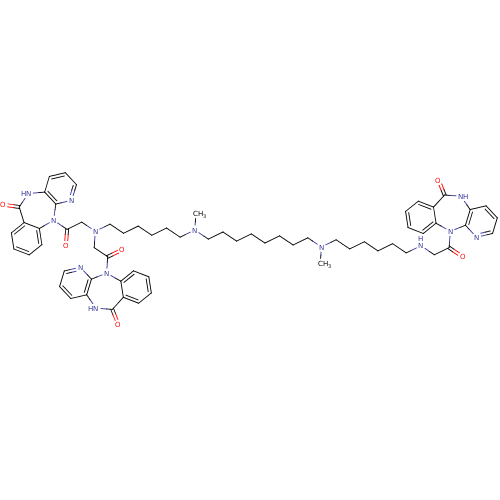
(CAS_132947 | NSC_132947 | TRIPITRAMINE)Show SMILES CN(CCCCCCCCN(C)CCCCCCN(CC(=O)N1c2ccccc2C(=O)Nc2cccnc12)CC(=O)N1c2ccccc2C(=O)Nc2cccnc12)CCCCCCNCC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C64H77N13O6/c1-72(41-20-8-5-17-35-65-44-56(78)75-53-32-14-11-26-47(53)62(81)69-50-29-23-36-66-59(50)75)39-18-6-3-4-7-19-40-73(2)42-21-9-10-22-43-74(45-57(79)76-54-33-15-12-27-48(54)63(82)70-51-30-24-37-67-60(51)76)46-58(80)77-55-34-16-13-28-49(55)64(83)71-52-31-25-38-68-61(52)77/h11-16,23-34,36-38,65H,3-10,17-22,35,39-46H2,1-2H3,(H,69,81)(H,70,82)(H,71,83) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 |
J Med Chem 41: 4150-60 (1998)
Checked by Author
Article DOI: 10.1021/jm981038d
BindingDB Entry DOI: 10.7270/Q2KH0PJH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50034875
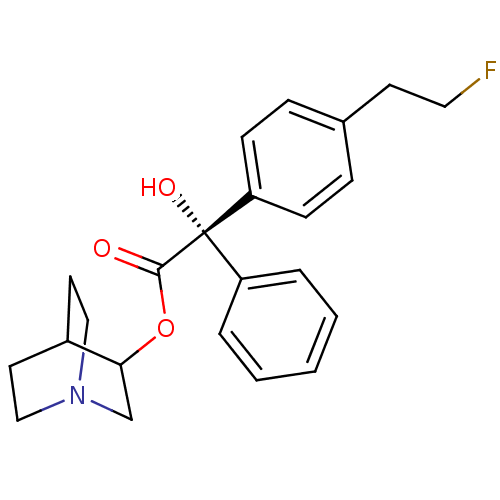
((S)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...)Show SMILES O[C@@](C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccc(CCF)cc1 |wU:1.1,wD:1.0,THB:4:5:9.8:11.12,(6.11,-5.14,;5.36,-6.48,;6.88,-7.04,;7.13,-8.54,;8.06,-6.06,;9.51,-6.59,;10.28,-5.37,;11.98,-4.6,;13.54,-5.53,;12.74,-6.67,;11.14,-5.72,;11.14,-4.34,;11.98,-3.53,;4.02,-5.71,;2.67,-6.48,;1.36,-5.71,;1.36,-4.17,;2.67,-3.38,;4.02,-4.15,;4.57,-7.81,;5.3,-9.12,;4.53,-10.44,;2.99,-10.44,;2.19,-11.77,;.65,-11.75,;-.12,-13.07,;2.22,-9.09,;3.03,-7.77,)| Show InChI InChI=1S/C23H26FNO3/c24-13-10-17-6-8-20(9-7-17)23(27,19-4-2-1-3-5-19)22(26)28-21-16-25-14-11-18(21)12-15-25/h1-9,18,21,27H,10-16H2/t21?,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 |
J Med Chem 38: 1711-9 (1995)
BindingDB Entry DOI: 10.7270/Q2PV6JDQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50230694
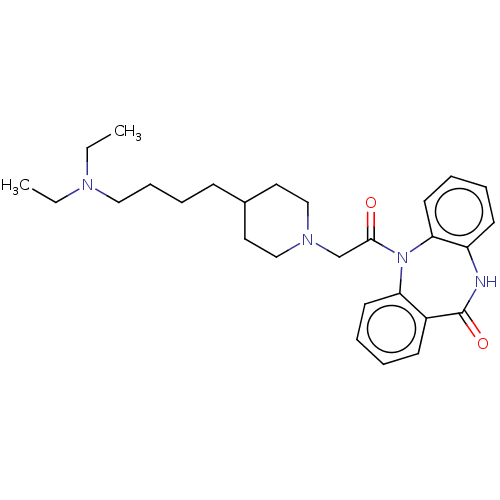
(CHEMBL295388)Show SMILES CCN(CC)CCCCC1CCN(CC(=O)N2c3ccccc3NC(=O)c3ccccc23)CC1 Show InChI InChI=1S/C28H38N4O2/c1-3-30(4-2)18-10-9-11-22-16-19-31(20-17-22)21-27(33)32-25-14-7-5-12-23(25)28(34)29-24-13-6-8-15-26(24)32/h5-8,12-15,22H,3-4,9-11,16-21H2,1-2H3,(H,29,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from rat heart muscarinic M2 receptor after 60 mins by scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM82372
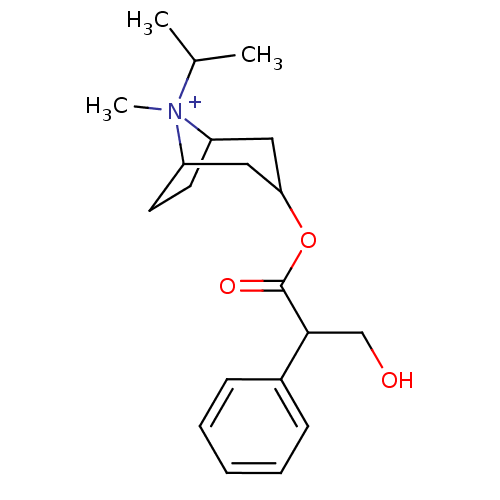
(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50408535
(CHEMBL131865)Show SMILES CN(CCCCCCN)CCCCCCCCN(C)CCCCCCNCCCC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C38H63N7O2/c1-43(31-17-9-5-13-25-39)29-15-7-3-4-8-16-30-44(2)32-18-10-6-14-26-40-27-20-24-36(46)45-35-23-12-11-21-33(35)38(47)42-34-22-19-28-41-37(34)45/h11-12,19,21-23,28,40H,3-10,13-18,20,24-27,29-32,39H2,1-2H3,(H,42,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 |
J Med Chem 41: 4150-60 (1998)
Checked by Author
Article DOI: 10.1021/jm981038d
BindingDB Entry DOI: 10.7270/Q2KH0PJH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50034872
((S)-[4-(3-Fluoro-propyl)-phenyl]-hydroxy-phenyl-ac...)Show SMILES O[C@@](C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccc(CCCF)cc1 |wU:1.1,wD:1.0,THB:4:5:9.8:11.12,(6.11,-5.14,;5.36,-6.48,;6.88,-7.04,;7.13,-8.54,;8.06,-6.06,;9.51,-6.59,;10.28,-5.37,;11.98,-4.6,;13.54,-5.53,;12.74,-6.67,;11.14,-5.72,;11.14,-4.34,;11.98,-3.53,;4.02,-5.71,;4.02,-4.15,;2.67,-3.38,;1.36,-4.17,;1.36,-5.71,;2.67,-6.48,;4.57,-7.81,;5.3,-9.12,;4.53,-10.44,;2.99,-10.44,;2.19,-11.77,;.65,-11.75,;-.12,-13.07,;-1.66,-13.06,;2.22,-9.09,;3.03,-7.77,)| Show InChI InChI=1S/C24H28FNO3/c25-14-4-5-18-8-10-21(11-9-18)24(28,20-6-2-1-3-7-20)23(27)29-22-17-26-15-12-19(22)13-16-26/h1-3,6-11,19,22,28H,4-5,12-17H2/t22?,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 |
J Med Chem 38: 1711-9 (1995)
BindingDB Entry DOI: 10.7270/Q2PV6JDQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50296314
((3R)-1-Azabicyclo[2.2.2]oct-3-yl9H-xanthene-9-carb...)Show SMILES O=C(O[C@H]1CN2CCC1CC2)C1c2ccccc2Oc2ccccc12 |r,wD:3.2,(-1.78,.66,;-3.13,-.09,;-3.15,-1.63,;-1.81,-2.4,;-1.81,-3.94,;-.48,-4.7,;.85,-3.94,;.85,-2.4,;-.48,-1.62,;-.06,-2.86,;-1.11,-3.21,;-4.45,.7,;-5.79,-.04,;-5.82,-1.58,;-7.17,-2.33,;-8.5,-1.53,;-8.47,.02,;-7.11,.76,;-7.09,2.3,;-5.74,3.04,;-5.72,4.57,;-4.38,5.32,;-3.05,4.53,;-3.07,2.99,;-4.42,2.25,)| Show InChI InChI=1S/C21H21NO3/c23-21(25-19-13-22-11-9-14(19)10-12-22)20-15-5-1-3-7-17(15)24-18-8-4-2-6-16(18)20/h1-8,14,19-20H,9-13H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121130
((2-Amino-3-methyl-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(C)c1N Show InChI InChI=1S/C34H41N3O6S/c1-24-4-3-5-31(32(24)35)33(38)37-20-16-27(17-21-37)36-18-14-26(15-19-36)34(42-22-23-43-34)25-6-10-29(11-7-25)44(39,40)30-12-8-28(41-2)9-13-30/h3-13,26-27H,14-23,35H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121134
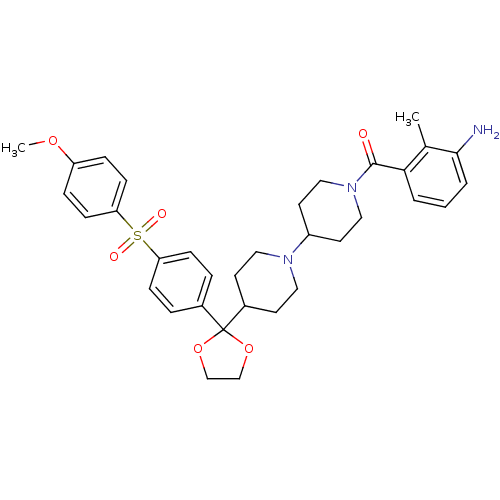
((3-Amino-2-methyl-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(N)c1C Show InChI InChI=1S/C34H41N3O6S/c1-24-31(4-3-5-32(24)35)33(38)37-20-16-27(17-21-37)36-18-14-26(15-19-36)34(42-22-23-43-34)25-6-10-29(11-7-25)44(39,40)30-12-8-28(41-2)9-13-30/h3-13,26-27H,14-23,35H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 71: 1725-9 (1974)
Article DOI: 10.1073/pnas.71.5.1725
BindingDB Entry DOI: 10.7270/Q2PC30WD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50369230
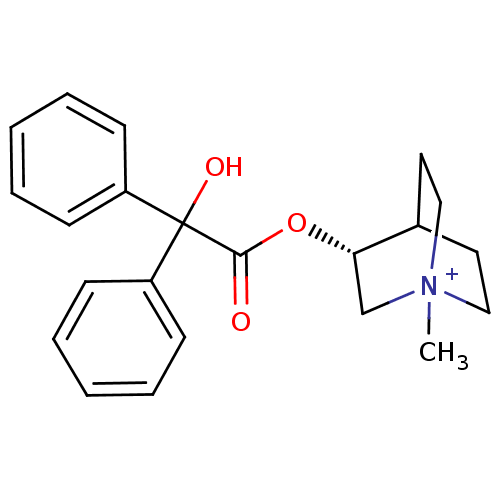
(CHEMBL1398637 | CLIDINIUM)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)C(O)(c1ccccc1)c1ccccc1 |wU:7.10,THB:9:7:2.3:6.5,(-1.62,-6.55,;-1.6,-5.01,;-3.17,-5.68,;-3.37,-4.27,;-1.87,-3.61,;-1.8,-1.94,;-1.34,-3.07,;-.49,-4.23,;-.2,-5.66,;.72,-3.27,;.49,-1.75,;-.95,-1.19,;1.69,-.79,;.73,.42,;2.9,.17,;2.67,1.69,;3.87,2.65,;5.3,2.09,;5.53,.57,;4.33,-.39,;2.65,-1.99,;4.17,-1.76,;5.13,-2.97,;4.57,-4.4,;3.05,-4.63,;2.09,-3.43,)| Show InChI InChI=1S/C22H26NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-11,17,20,25H,12-16H2,1H3/q+1/t17?,20-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50408530
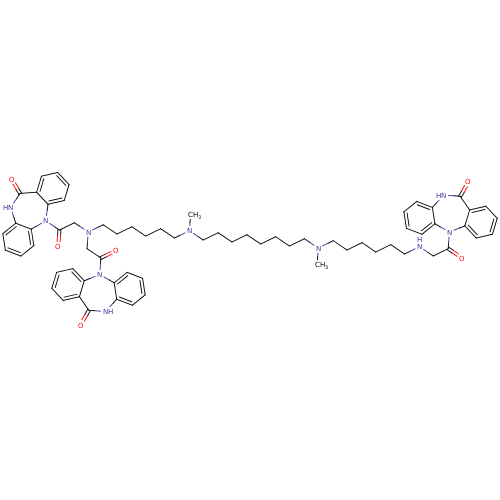
(CHEMBL1202003)Show SMILES CN(CCCCCCCCN(C)CCCCCCN(CC(=O)N1c2ccccc2NC(=O)c2ccccc12)CC(=O)N1c2ccccc2NC(=O)c2ccccc12)CCCCCCNCC(=O)N1c2ccccc2NC(=O)c2ccccc12 Show InChI InChI=1S/C67H80N10O6/c1-72(44-26-8-5-23-41-68-47-62(78)75-56-35-17-11-29-50(56)65(81)69-53-32-14-20-38-59(53)75)42-24-6-3-4-7-25-43-73(2)45-27-9-10-28-46-74(48-63(79)76-57-36-18-12-30-51(57)66(82)70-54-33-15-21-39-60(54)76)49-64(80)77-58-37-19-13-31-52(58)67(83)71-55-34-16-22-40-61(55)77/h11-22,29-40,68H,3-10,23-28,41-49H2,1-2H3,(H,69,81)(H,70,82)(H,71,83) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 |
J Med Chem 41: 4150-60 (1998)
Checked by Author
Article DOI: 10.1021/jm981038d
BindingDB Entry DOI: 10.7270/Q2KH0PJH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121126

((2-Amino-4-fluoro-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1ccc(F)cc1N Show InChI InChI=1S/C33H38FN3O6S/c1-41-27-5-9-29(10-6-27)44(39,40)28-7-2-23(3-8-28)33(42-20-21-43-33)24-12-16-36(17-13-24)26-14-18-37(19-15-26)32(38)30-11-4-25(34)22-31(30)35/h2-11,22,24,26H,12-21,35H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021904
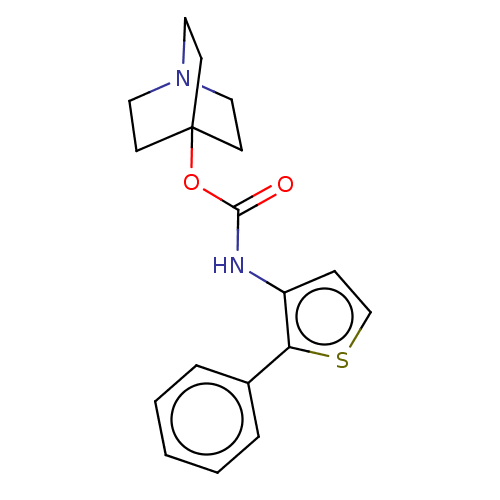
(CHEMBL3298588)Show InChI InChI=1S/C18H20N2O2S.ClH/c21-17(22-18-7-10-20(11-8-18)12-9-18)19-15-6-13-23-16(15)14-4-2-1-3-5-14;/h1-6,13H,7-12H2,(H,19,21);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Division of American Cyanamid Company
Curated by ChEMBL
| Assay Description
Binding affinity at [3H]QNB and GppNHp radiolabeled muscarinic M2 receptor in rat heart. |
J Med Chem 36: 3533-41 (1994)
BindingDB Entry DOI: 10.7270/Q28916GB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Italia
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 352: 276-82 (1995)
Article DOI: 10.1007/bf00168557
BindingDB Entry DOI: 10.7270/Q2Q52N48 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Division of American Cyanamid Company
Curated by ChEMBL
| Assay Description
Binding affinity at [3H]CD radiolabeled muscarinic M2 receptor in rat cortex. |
J Med Chem 36: 3533-41 (1994)
BindingDB Entry DOI: 10.7270/Q28916GB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121138
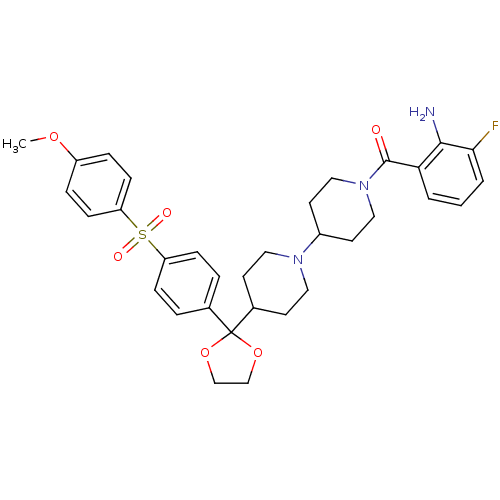
((2-Amino-3-fluoro-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(F)c1N Show InChI InChI=1S/C33H38FN3O6S/c1-41-26-7-11-28(12-8-26)44(39,40)27-9-5-23(6-10-27)33(42-21-22-43-33)24-13-17-36(18-14-24)25-15-19-37(20-16-25)32(38)29-3-2-4-30(34)31(29)35/h2-12,24-25H,13-22,35H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121139
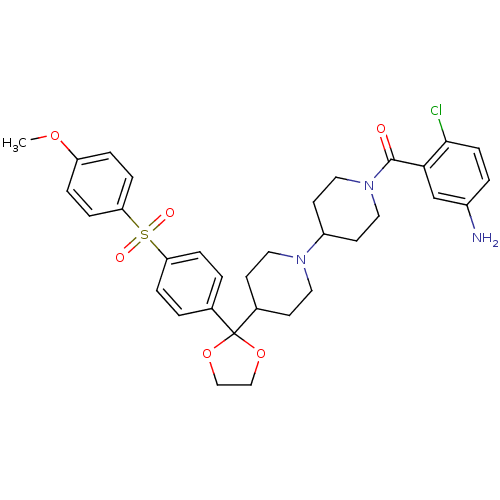
((5-Amino-2-chloro-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cc(N)ccc1Cl Show InChI InChI=1S/C33H38ClN3O6S/c1-41-27-5-9-29(10-6-27)44(39,40)28-7-2-23(3-8-28)33(42-20-21-43-33)24-12-16-36(17-13-24)26-14-18-37(19-15-26)32(38)30-22-25(35)4-11-31(30)34/h2-11,22,24,26H,12-21,35H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121127
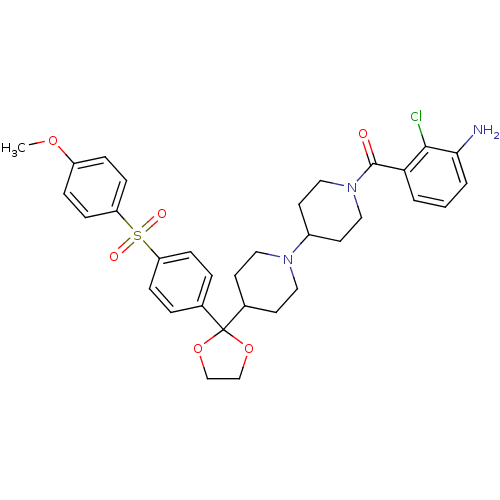
((3-Amino-2-chloro-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(N)c1Cl Show InChI InChI=1S/C33H38ClN3O6S/c1-41-26-7-11-28(12-8-26)44(39,40)27-9-5-23(6-10-27)33(42-21-22-43-33)24-13-17-36(18-14-24)25-15-19-37(20-16-25)32(38)29-3-2-4-30(35)31(29)34/h2-12,24-25H,13-22,35H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121124
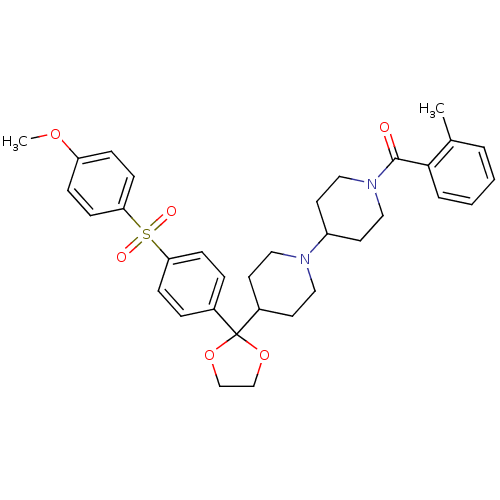
((4-{2-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-[1,3]...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1ccccc1C Show InChI InChI=1S/C34H40N2O6S/c1-25-5-3-4-6-32(25)33(37)36-21-17-28(18-22-36)35-19-15-27(16-20-35)34(41-23-24-42-34)26-7-11-30(12-8-26)43(38,39)31-13-9-29(40-2)10-14-31/h3-14,27-28H,15-24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
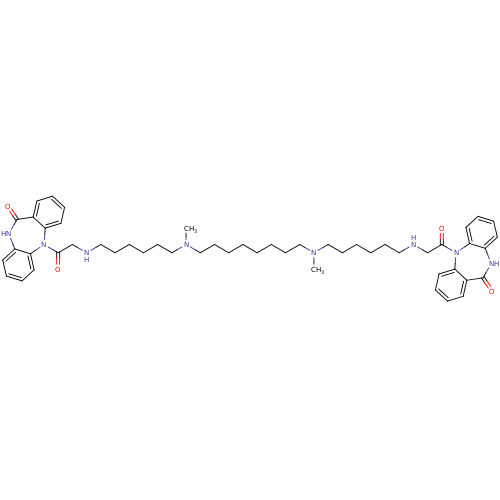
(RAT) | BDBM50408527
(CHEMBL1202004)Show SMILES CN(CCCCCCCCN(C)CCCCCCNCC(=O)N1c2ccccc2NC(=O)c2ccccc12)CCCCCCNCC(=O)N1c2ccccc2NC(=O)c2ccccc12 Show InChI InChI=1S/C52H70N8O4/c1-57(37-23-9-5-19-33-53-39-49(61)59-45-29-15-11-25-41(45)51(63)55-43-27-13-17-31-47(43)59)35-21-7-3-4-8-22-36-58(2)38-24-10-6-20-34-54-40-50(62)60-46-30-16-12-26-42(46)52(64)56-44-28-14-18-32-48(44)60/h11-18,25-32,53-54H,3-10,19-24,33-40H2,1-2H3,(H,55,63)(H,56,64) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 |
J Med Chem 41: 4150-60 (1998)
Checked by Author
Article DOI: 10.1021/jm981038d
BindingDB Entry DOI: 10.7270/Q2KH0PJH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute for Medical Research
Curated by PDSP Ki Database
| |
Annu Rev Pharmacol Toxicol 30: 633-73 (1990)
Article DOI: 10.1146/annurev.pa.30.040190.003221
BindingDB Entry DOI: 10.7270/Q21Z42WK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021938
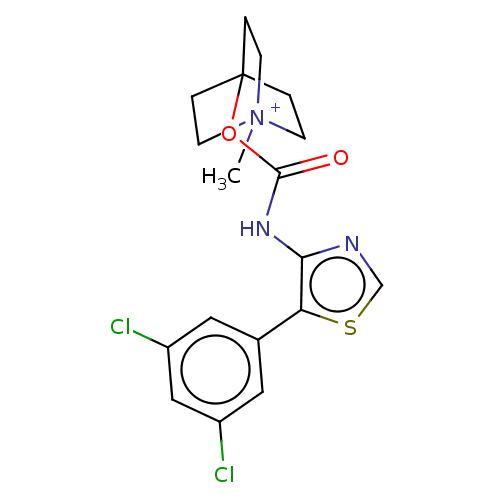
(CHEMBL3298763)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C18H19Cl2N3O2S.HI/c1-23-5-2-18(3-6-23,4-7-23)25-17(24)22-16-15(26-11-21-16)12-8-13(19)10-14(20)9-12;/h8-11H,2-7H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50018550
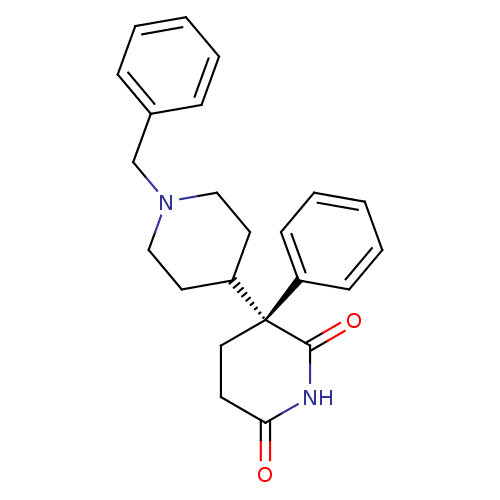
(1'-Benzyl-3-phenyl-[3,4']bipiperidinyl-2,6-dione |...)Show SMILES O=C1CC[C@](C2CCN(Cc3ccccc3)CC2)(C(=O)N1)c1ccccc1 Show InChI InChI=1S/C23H26N2O2/c26-21-11-14-23(22(27)24-21,19-9-5-2-6-10-19)20-12-15-25(16-13-20)17-18-7-3-1-4-8-18/h1-10,20H,11-17H2,(H,24,26,27)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121125
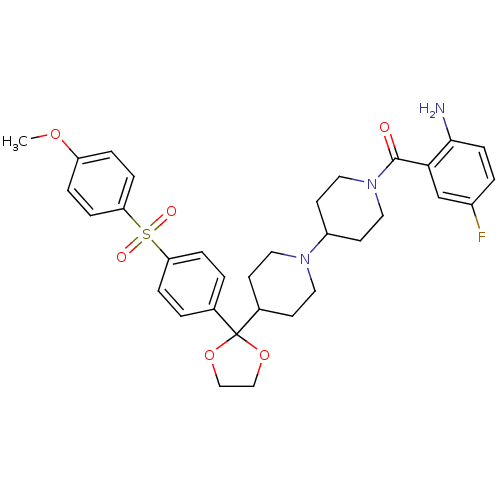
((2-Amino-5-fluoro-phenyl)-(4-{2-[4-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cc(F)ccc1N Show InChI InChI=1S/C33H38FN3O6S/c1-41-27-5-9-29(10-6-27)44(39,40)28-7-2-23(3-8-28)33(42-20-21-43-33)24-12-16-36(17-13-24)26-14-18-37(19-15-26)32(38)30-22-25(34)4-11-31(30)35/h2-11,22,24,26H,12-21,35H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403547
(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium |
J Med Chem 31: 1463-6 (1988)
BindingDB Entry DOI: 10.7270/Q2DV1KGN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121131
((3-Chloro-2-methyl-phenyl)-(4-{2-[4-(4-methoxy-ben...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1cccc(Cl)c1C Show InChI InChI=1S/C34H39ClN2O6S/c1-24-31(4-3-5-32(24)35)33(38)37-20-16-27(17-21-37)36-18-14-26(15-19-36)34(42-22-23-43-34)25-6-10-29(11-7-25)44(39,40)30-12-8-28(41-2)9-13-30/h3-13,26-27H,14-23H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-quinuclidinyl benzilate(QNB) from muscarinic (M2) receptor in rat heart homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data