

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50280622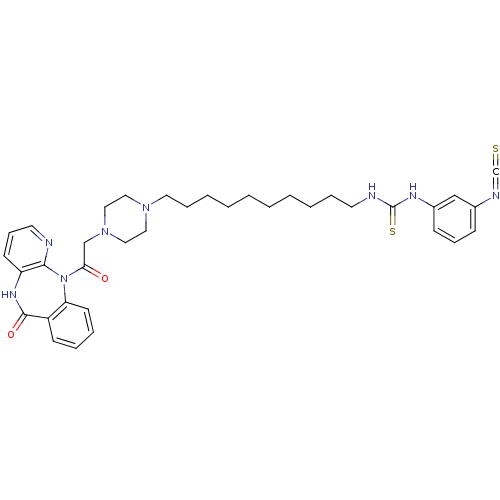 (1-(3-Isothiocyanato-phenyl)-3-(4-{4-[2-oxo-2-(6-ox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated for irreversible inhibition at rat forebrain muscarinic receptor | Bioorg Med Chem Lett 2: 845-850 (1992) Article DOI: 10.1016/S0960-894X(00)80542-9 BindingDB Entry DOI: 10.7270/Q2F18ZMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062577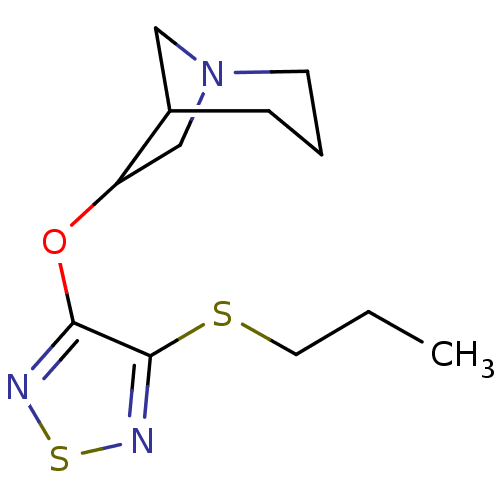 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033155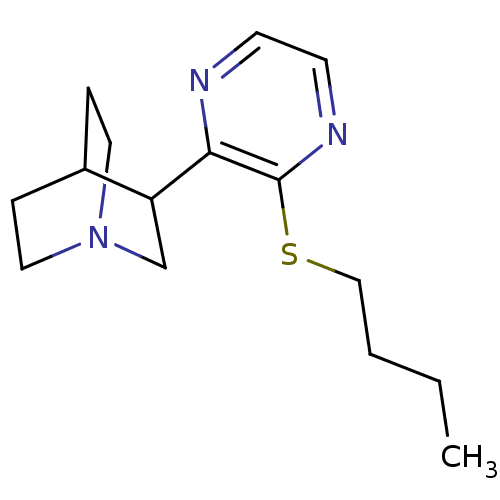 (3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033155 (3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062577 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062577 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062595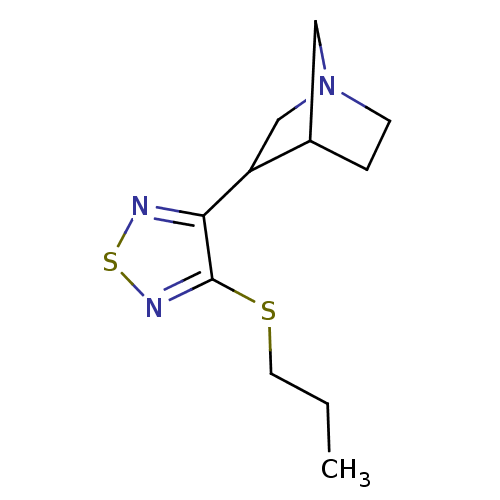 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-aza-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062595 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-aza-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033162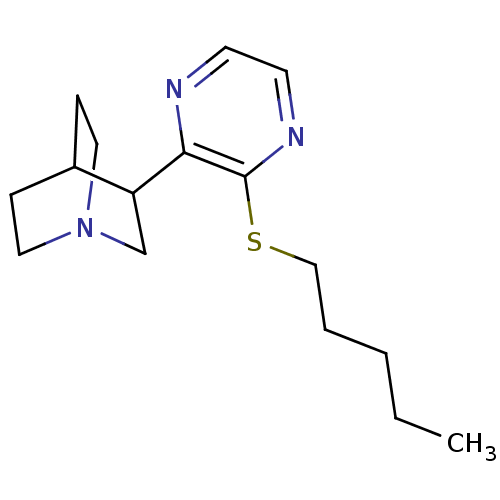 (3-(3-Pentylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062570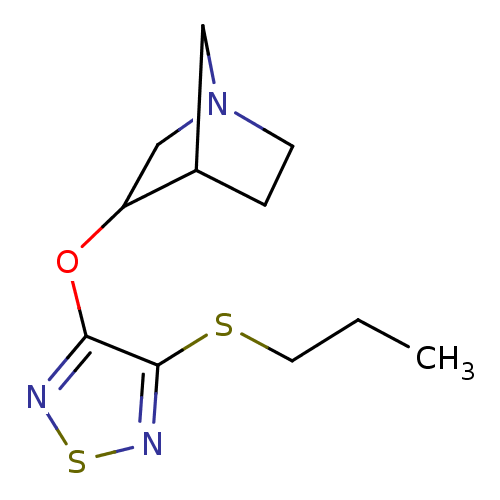 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006582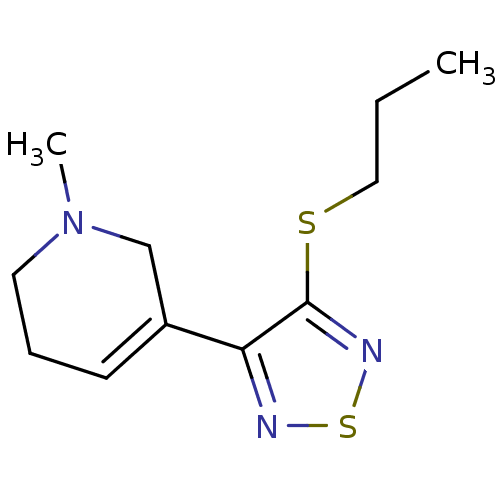 (1-Methyl-5-(4-propylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033162 (3-(3-Pentylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006572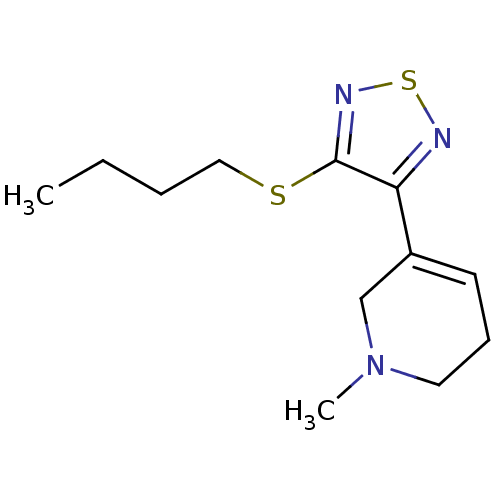 (5-(4-Butylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-methy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006572 (5-(4-Butylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-methy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351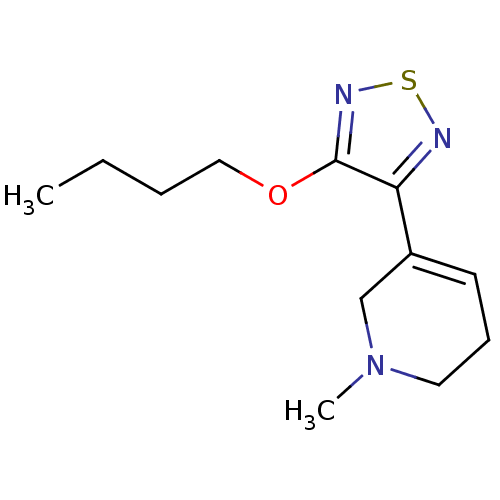 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-oxotremorine-M (Oxo-M) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003369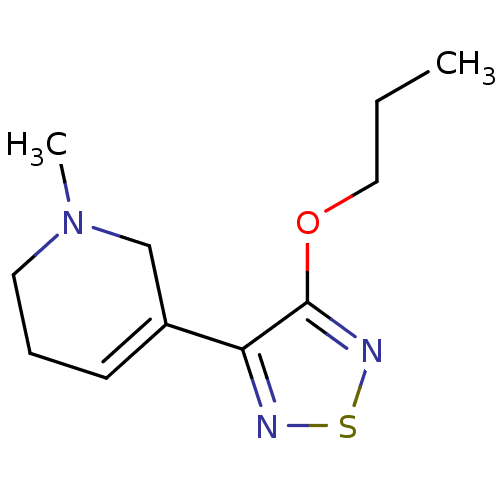 (1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against rat hippocampus Muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003369 (1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033151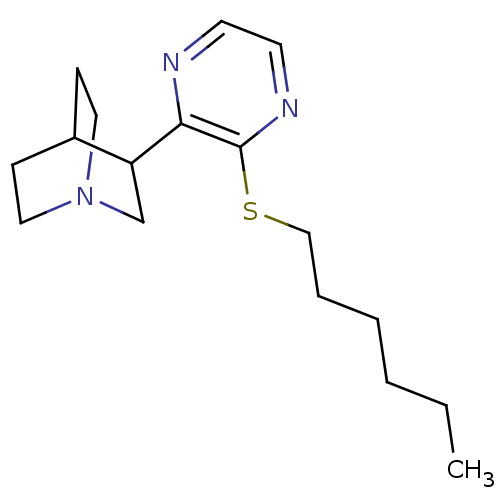 (3-(3-Hexylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50605085 (CHEMBL5204021) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033151 (3-(3-Hexylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006580 (1-Methyl-5-(4-methylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003363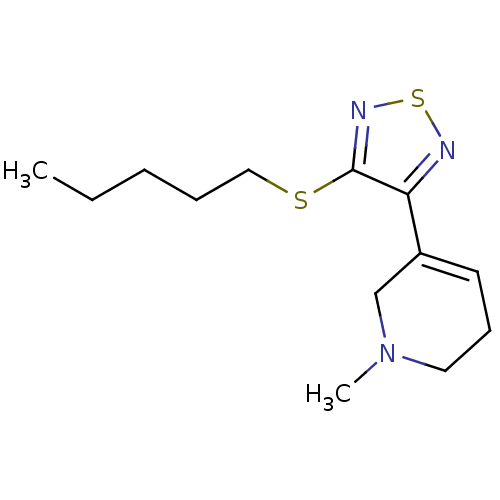 (1-Methyl-5-(4-pentylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-oxotremorine-M (Oxo-M) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453786 (CHEMBL2092949) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062589 (3-(4-Butylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033152 ((R)-6-(3-Hexyloxy-pyrazin-2-yl)-1-aza-bicyclo[3.2....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062585 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453786 (CHEMBL2092949) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche - Università di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-pirenzepine binding to muscarinic M1 receptor in brain cortex of rat. | Bioorg Med Chem Lett 9: 3031-4 (1999) BindingDB Entry DOI: 10.7270/Q2V40TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006582 (1-Methyl-5-(4-propylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033149 (3-Pyrazin-2-yl-1-aza-bicyclo[2.2.2]octane | 3-Pyra...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033152 ((R)-6-(3-Hexyloxy-pyrazin-2-yl)-1-aza-bicyclo[3.2....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003366 (1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-pirenzepine (Pz) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003366 (1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-pirenzepine (Pz) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003366 (1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003366 (1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062570 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062579 (3-(4-Pentylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062570 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033148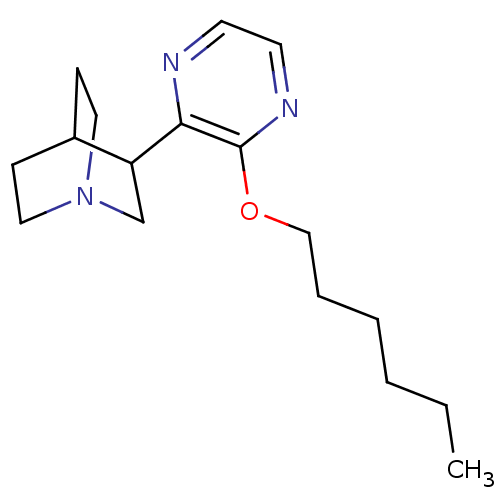 (3-(3-Hexyloxy-pyrazin-2-yl)-1-aza-bicyclo[2.2.2]oc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033163 (3-(6-Chloro-pyrazin-2-yl)-1-aza-bicyclo[2.2.2]octa...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033163 (3-(6-Chloro-pyrazin-2-yl)-1-aza-bicyclo[2.2.2]octa...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003363 (1-Methyl-5-(4-pentylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against M1 receptor from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand. | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003363 (1-Methyl-5-(4-pentylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-oxotremorine-M (Oxo-M) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003363 (1-Methyl-5-(4-pentylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-Pz (pirenzepine) from the muscarinic receptor M1 of the rat hippocampus | Bioorg Med Chem Lett 2: 809-814 (1992) Article DOI: 10.1016/S0960-894X(00)80536-3 BindingDB Entry DOI: 10.7270/Q2T72HBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50283129 (5-(4-Hex-3-ynyloxy-[1,2,5]thiadiazol-3-yl)-1,2-dim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards Muscarinic acetylcholine receptor M1 by the displacement of [3H]-pirenzepine in rat cerebral cortical membranes | Bioorg Med Chem Lett 4: 2205-2210 (1994) Article DOI: 10.1016/S0960-894X(00)80072-4 BindingDB Entry DOI: 10.7270/Q2T72HCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 485 total ) | Next | Last >> |