 Found 7 hits of ki for UniProtKB: P08911
Found 7 hits of ki for UniProtKB: P08911 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50403547
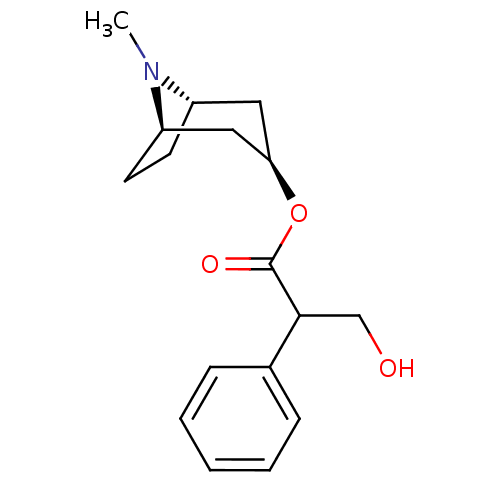
(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M5 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 885-90 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.051
BindingDB Entry DOI: 10.7270/Q2DZ095Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50165008
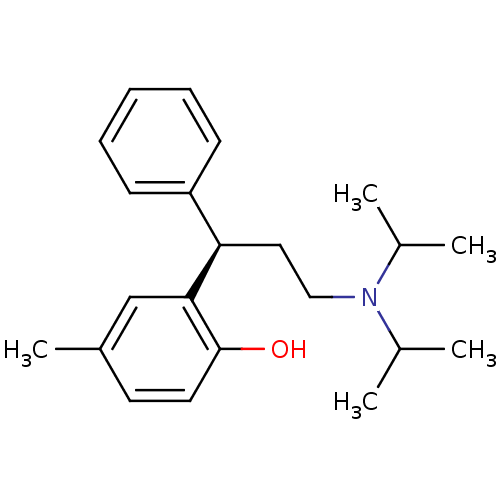
((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50374004
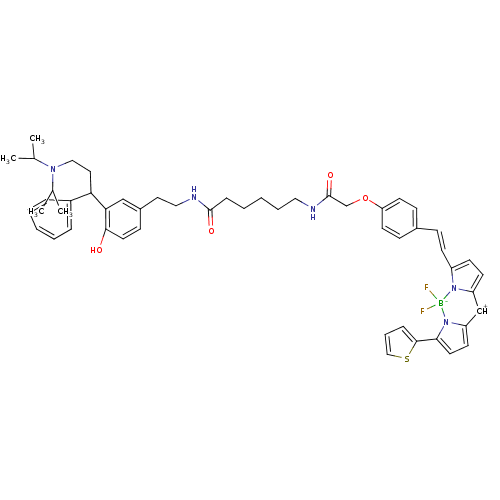
(CHEMBL271108)Show SMILES CC(C)N(CCC(c1ccccc1)c1cc(CCNC(=O)CCCCCNC(=O)COc2ccc([CH+]\C=c3/ccc4=Cc5ccc(-c6cccs6)n5[B-](F)(F)n34)cc2)ccc1O)C(C)C |t:41| Show InChI InChI=1S/C52H60BF2N5O4S/c1-37(2)58(38(3)4)32-29-46(41-12-7-5-8-13-41)47-34-40(19-27-49(47)61)28-31-57-51(62)15-9-6-10-30-56-52(63)36-64-45-24-17-39(18-25-45)16-20-42-21-22-43-35-44-23-26-48(50-14-11-33-65-50)60(44)53(54,55)59(42)43/h5,7-8,11-14,16-27,33-35,37-38,46,61H,6,9-10,15,28-32,36H2,1-4H3,(H,56,63)(H,57,62)/b42-20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50605085

(CHEMBL5204021)Show SMILES Cn1ccnc1SC[C@H]1CCN(C[C@@H]1F)S(=O)(=O)c1ccc2OCCc2c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00192
BindingDB Entry DOI: 10.7270/Q23N27HG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50231845
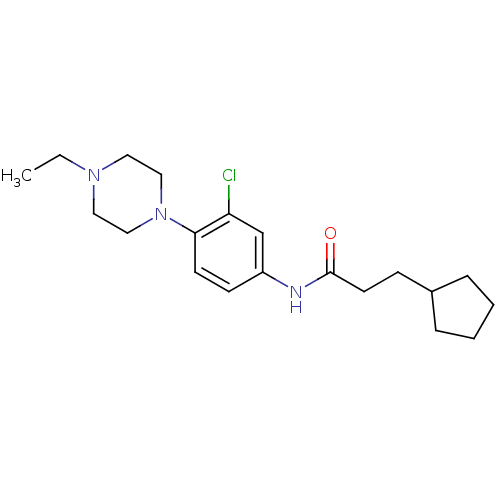
(CHEMBL255523 | N-(3-chloro-4-(4-ethylpiperazin-1-y...)Show InChI InChI=1S/C20H30ClN3O/c1-2-23-11-13-24(14-12-23)19-9-8-17(15-18(19)21)22-20(25)10-7-16-5-3-4-6-16/h8-9,15-16H,2-7,10-14H2,1H3,(H,22,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat muscarinic M5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 885-90 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.051
BindingDB Entry DOI: 10.7270/Q2DZ095Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data