

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (RAT) | BDBM50105051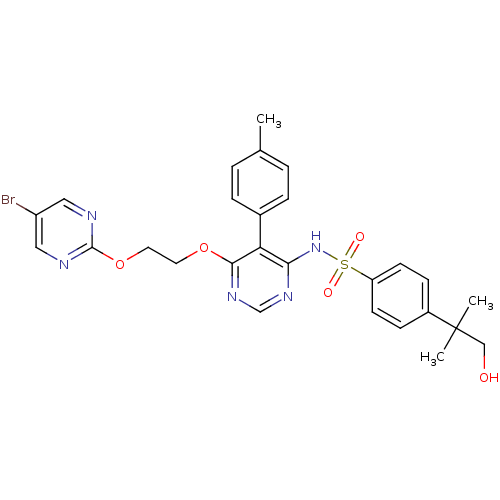 (CHEMBL112624 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of 125I-ET1 from ETA receptor in rat A7R5 cells | Bioorg Med Chem Lett 26: 3381-94 (2016) Article DOI: 10.1016/j.bmcl.2016.06.014 BindingDB Entry DOI: 10.7270/Q26113SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50105051 (CHEMBL112624 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of 125I-ET1 from ETA receptor in rat A7R5 cells | Bioorg Med Chem Lett 26: 3381-94 (2016) Article DOI: 10.1016/j.bmcl.2016.06.014 BindingDB Entry DOI: 10.7270/Q26113SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143792 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143796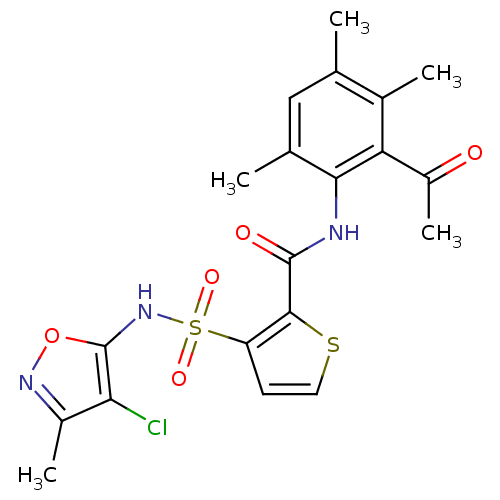 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50532607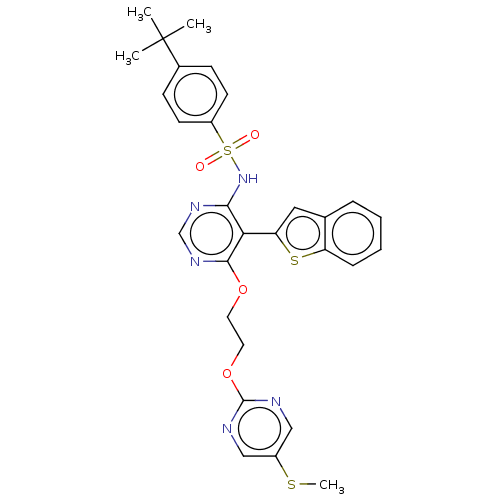 (CHEMBL4538570) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of 125I-ET1 from ETA receptor in rat A7R5 cells | Bioorg Med Chem Lett 26: 3381-94 (2016) Article DOI: 10.1016/j.bmcl.2016.06.014 BindingDB Entry DOI: 10.7270/Q26113SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50532607 (CHEMBL4538570) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of 125I-ET1 from ETA receptor in rat A7R5 cells | Bioorg Med Chem Lett 26: 3381-94 (2016) Article DOI: 10.1016/j.bmcl.2016.06.014 BindingDB Entry DOI: 10.7270/Q26113SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143802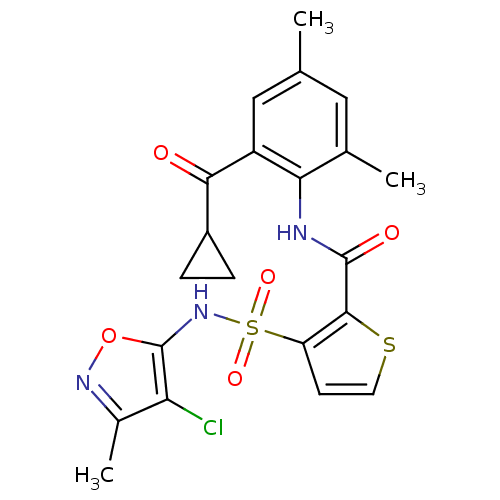 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143797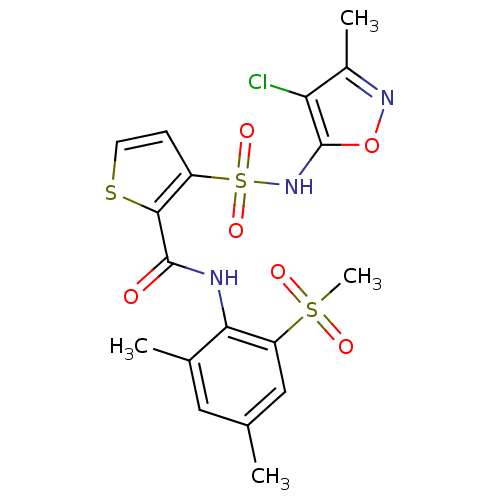 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143786 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070888 (CHEMBL298725 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50369954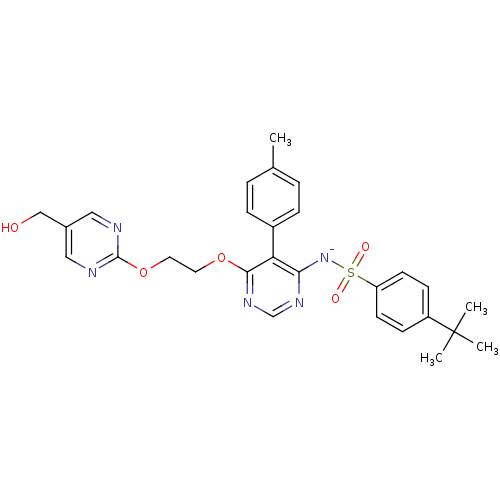 (CHEMBL1627023) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to endothelin A receptor in porcine aortic membrane | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50104995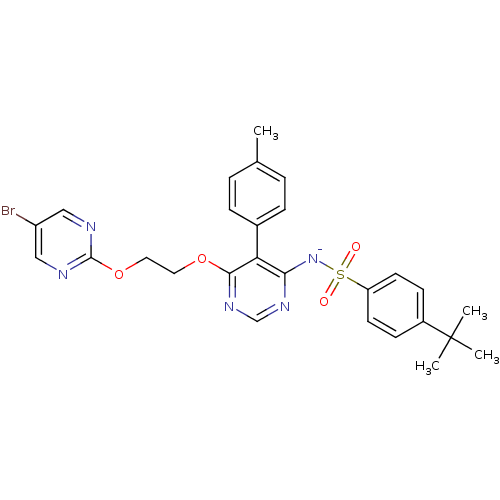 (CHEMBL369489 | sodium salt of N-{6-[2-(5-Bromo-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50105033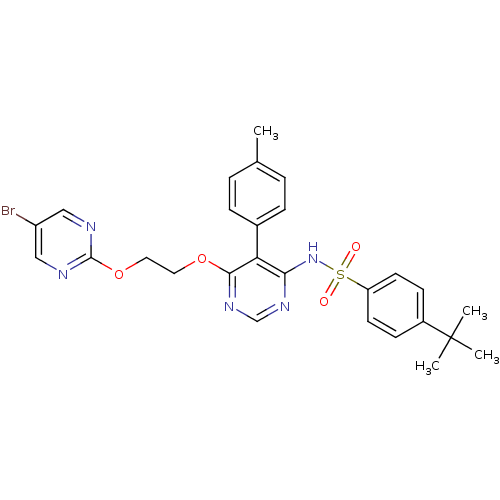 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit specific binding of [125I]- -ET-1 to rat A 10 cells which express endothelin A receptor | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143783 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50061096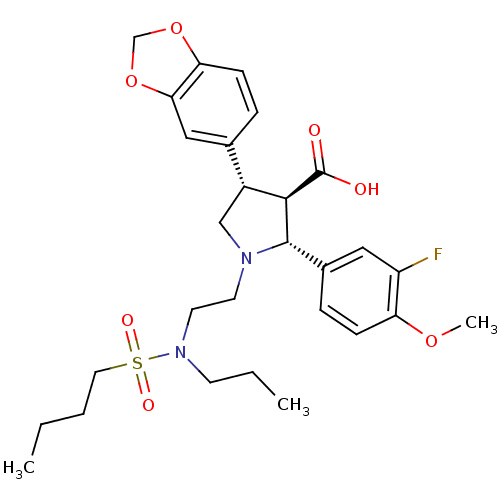 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143778 (2-{[3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50098777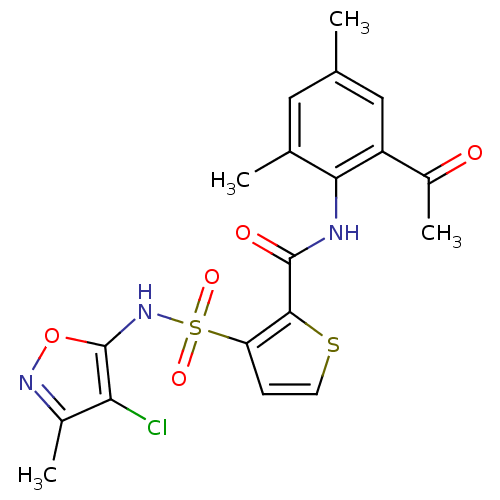 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50146613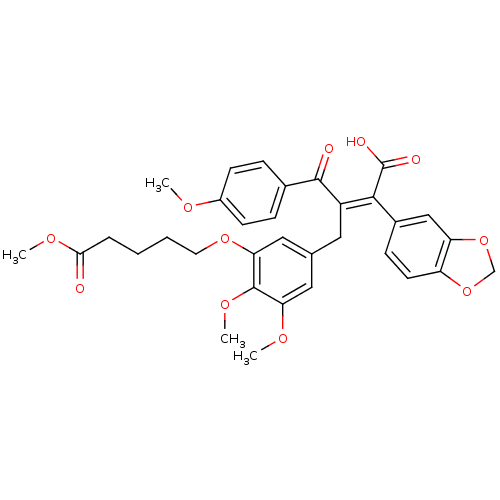 (5-{5-[(Z)-3-Benzo[1,3]dioxol-5-yl-3-carboxy-2-(4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143789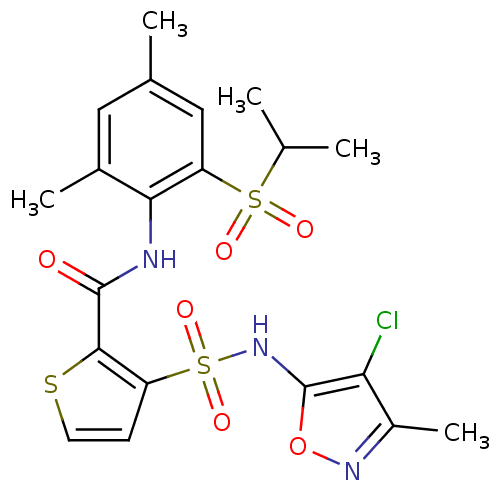 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143773 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143801 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143785 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143788 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50106408 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor in MMQ cells in rat | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50105027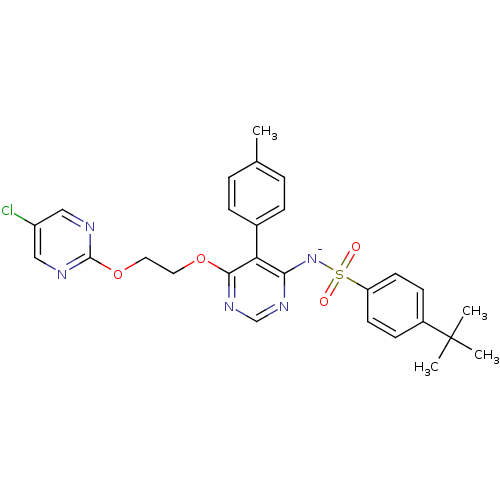 (CHEMBL176272 | sodium salt of 4-tert-Butyl-N-{6-[2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143787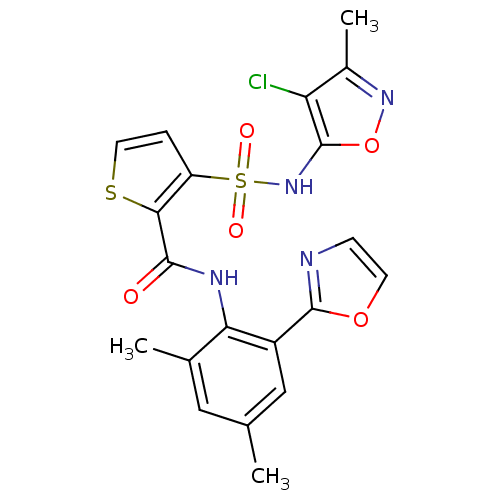 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143780 (3-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-thiophene-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143793 (3-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-thiophene-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50051007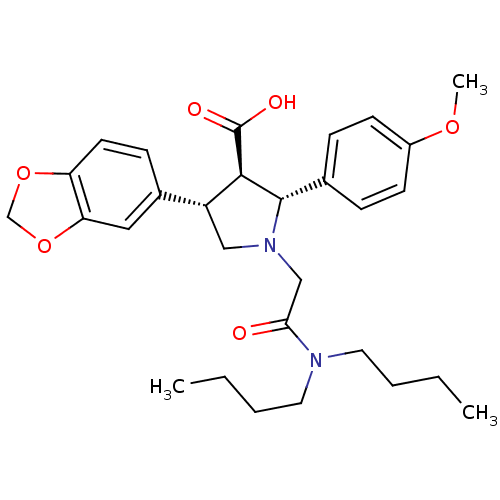 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Endothelin A receptor | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143794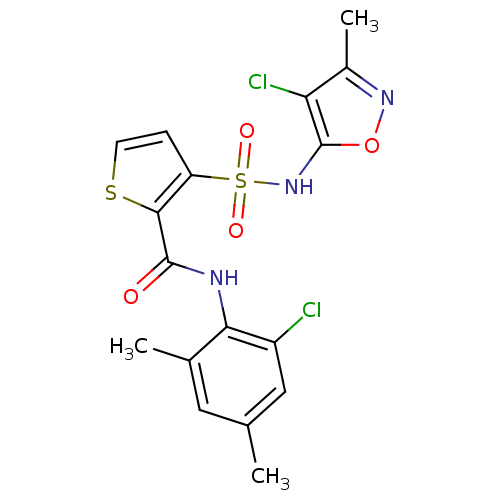 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50061086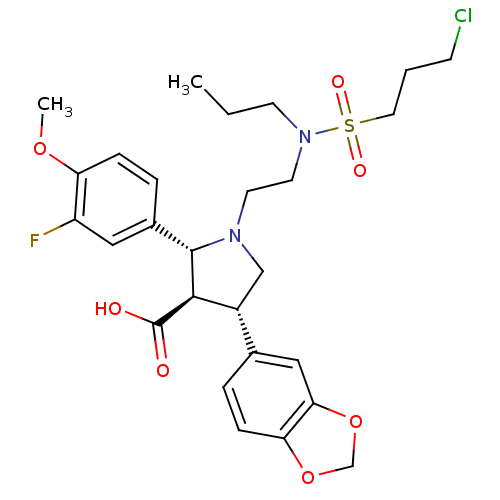 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(3-chloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to displace endothelin ([125I]-ET-1) from endothelin A receptor derived from MMQ cells of rodent. | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50061096 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to displace endothelin ([125I]-ET-1) from endothelin A receptor derived from MMQ cells of rodent. | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143774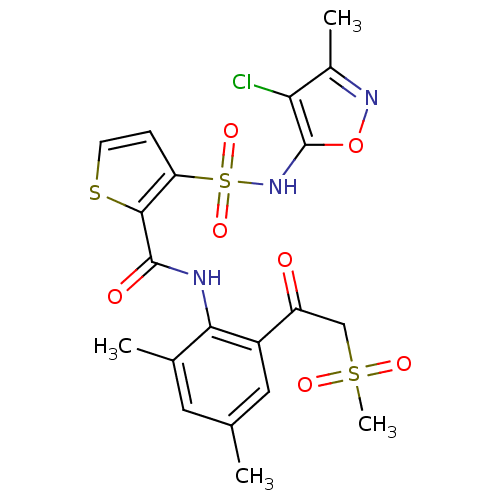 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50106403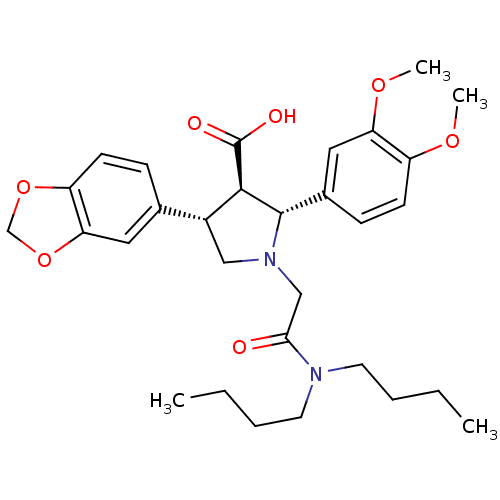 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor in MMQ cells in rat | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143772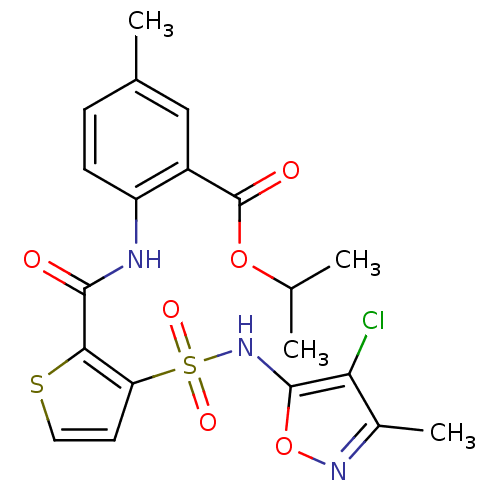 (2-{[3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50146606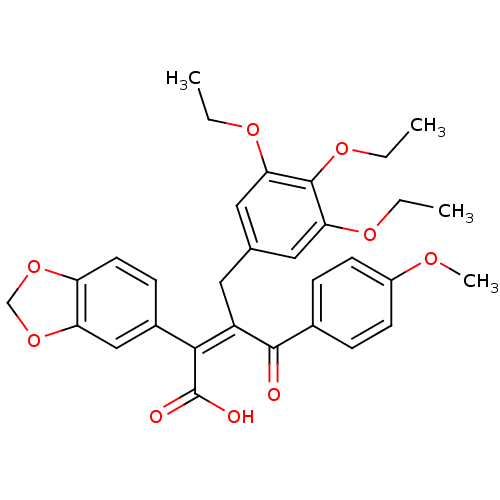 ((Z)-2-Benzo[1,3]dioxol-5-yl-4-(4-methoxy-phenyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50106400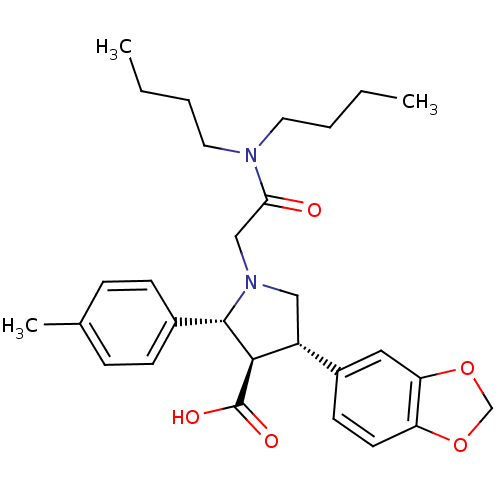 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor in MMQ cells in rat | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143790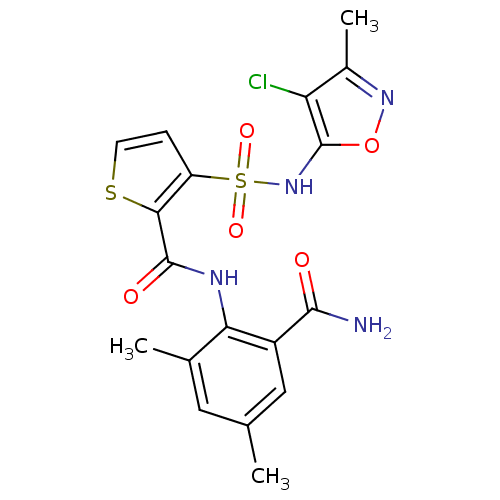 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50098772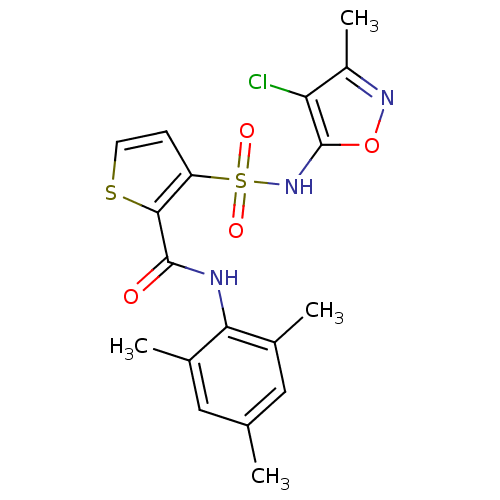 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50106402 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor in MMQ cells in rat | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50112678 ((R)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50112678 ((R)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for endothelin A receptor by inhibition of [125I]-ET-1 binding in rat aorta smooth muscle cells | J Med Chem 45: 2041-55 (2002) BindingDB Entry DOI: 10.7270/Q24J0DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50112678 ((R)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) Curated by ChEMBL | Assay Description Displacement of [125I]ET-1 from ET-A receptor in RASMC incubated for 60 mins | J Med Chem 59: 8168-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01781 BindingDB Entry DOI: 10.7270/Q22N55RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50112738 (2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for endothelin A receptor by inhibition of [125I]-ET-1 binding in rat aorta smooth muscle cells | J Med Chem 45: 2041-55 (2002) BindingDB Entry DOI: 10.7270/Q24J0DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143781 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-5-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50061099 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-2-(3-fluoro-4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to displace endothelin ([125I]-ET-1) from endothelin A receptor derived from MMQ cells of rodent. | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50050975 ((2S,3S,4R)-4-Benzo[1,3]dioxol-5-yl-1-[(butyl-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against the Endothelin A receptor of rat MMQ cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143777 (CHEMBL61425 | TAK-044 | {(2S,5S,8S,11R,14R,17S)-14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration towards endothelin A receptor in rat. | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50104987 (CHEMBL367445 | sodium salt of 4-tert-Butyl-N-{6-[2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50112662 (2-Benzo[1,3]dioxol-5-yl-4-butylsulfanyl-6-isopropo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for endothelin A receptor by inhibition of [125I]-ET-1 binding in rat aorta smooth muscle cells | J Med Chem 45: 2041-55 (2002) BindingDB Entry DOI: 10.7270/Q24J0DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 940 total ) | Next | Last >> |