 Found 86 hits of ec50 data for polymerid = 49000646
Found 86 hits of ec50 data for polymerid = 49000646 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414494

(CHEMBL564300)Show InChI InChI=1S/C14H13N3O2S2/c18-14(19)12-5-13(16-15-12)17(6-10-1-3-20-8-10)7-11-2-4-21-9-11/h1-5,8-9H,6-7H2,(H,15,16)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414492
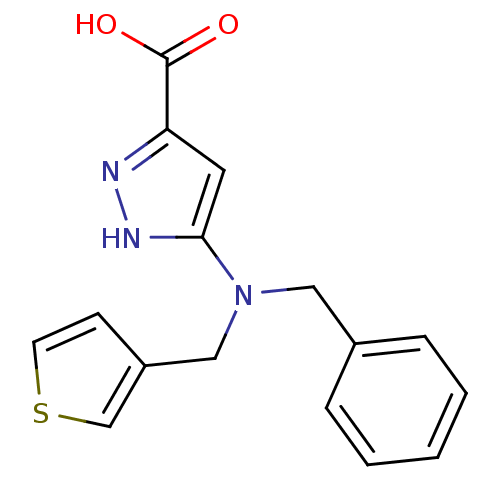
(CHEMBL564914)Show InChI InChI=1S/C16H15N3O2S/c20-16(21)14-8-15(18-17-14)19(10-13-6-7-22-11-13)9-12-4-2-1-3-5-12/h1-8,11H,9-10H2,(H,17,18)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414513

(CHEMBL394468)Show InChI InChI=1S/C9H10N2O2/c1-2-5-10-8-4-3-7(6-11-8)9(12)13/h2-4,6H,1,5H2,(H,10,11)(H,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384637
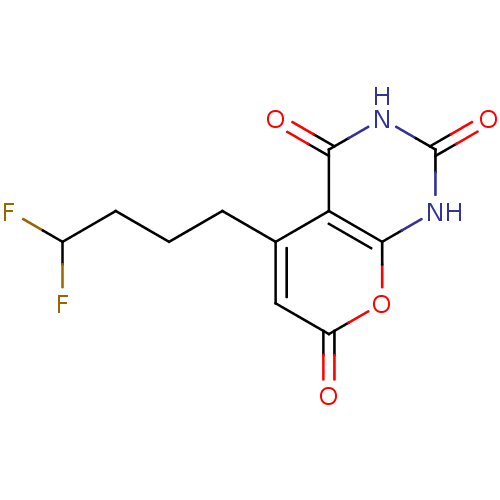
(CHEMBL2036951)Show InChI InChI=1S/C11H10F2N2O4/c12-6(13)3-1-2-5-4-7(16)19-10-8(5)9(17)14-11(18)15-10/h4,6H,1-3H2,(H2,14,15,17,18) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384640
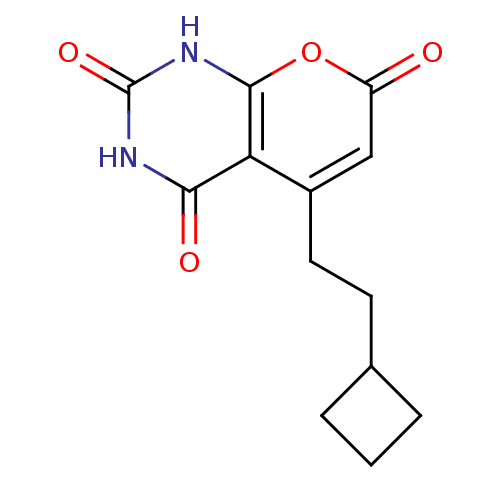
(CHEMBL2036954)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(5-4-7-2-1-3-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384612
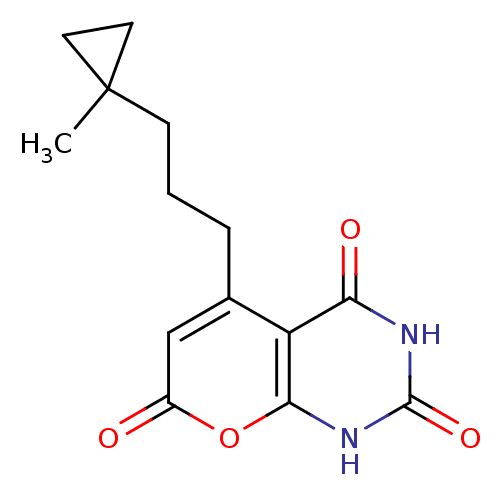
(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384641
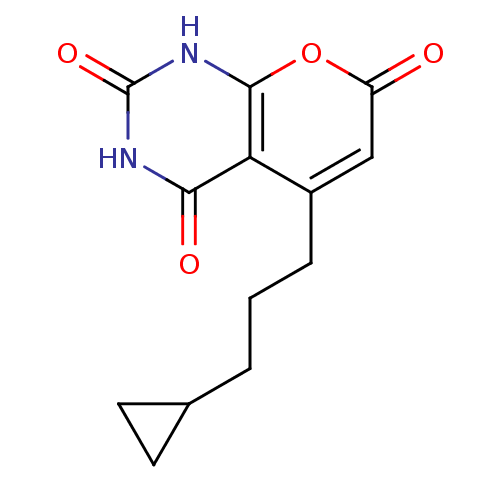
(CHEMBL2036955)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(3-1-2-7-4-5-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414491
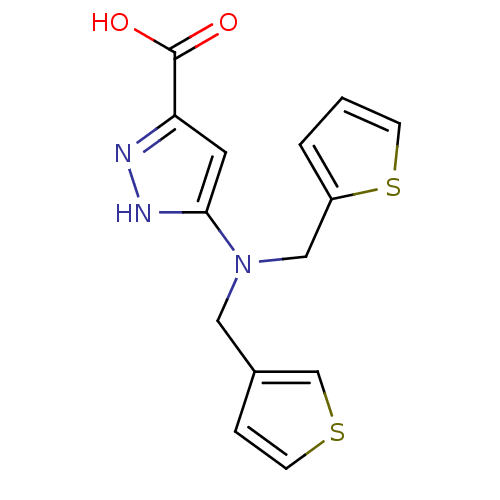
(CHEMBL559204)Show InChI InChI=1S/C14H13N3O2S2/c18-14(19)12-6-13(16-15-12)17(7-10-3-5-20-9-10)8-11-2-1-4-21-11/h1-6,9H,7-8H2,(H,15,16)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208135
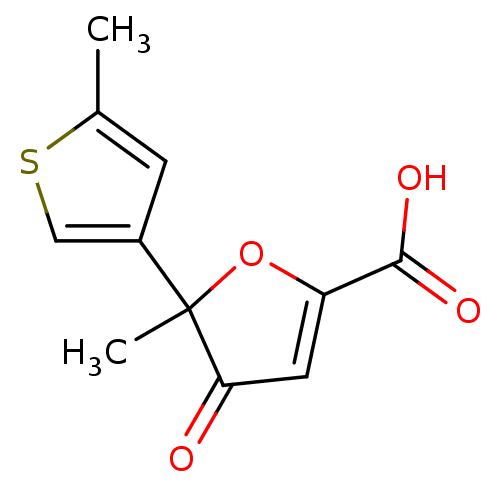
((+)-5-methyl-5-(5-methyl-thiophen-3-yl)-4-oxo-4,5-...)Show InChI InChI=1S/C11H10O4S/c1-6-3-7(5-16-6)11(2)9(12)4-8(15-11)10(13)14/h3-5H,1-2H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384616
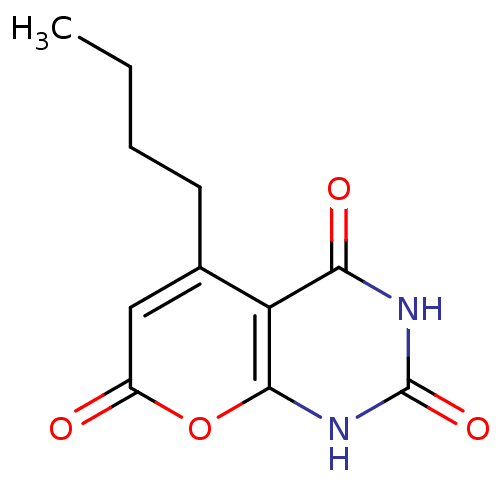
(CHEMBL2036813)Show InChI InChI=1S/C11H12N2O4/c1-2-3-4-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475630

(CHEMBL382096)Show InChI InChI=1S/C11H13N3O3/c1-7(6-17-2)14-10-4-3-8(11(15)16)5-9(10)12-13-14/h3-5,7H,6H2,1-2H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475626
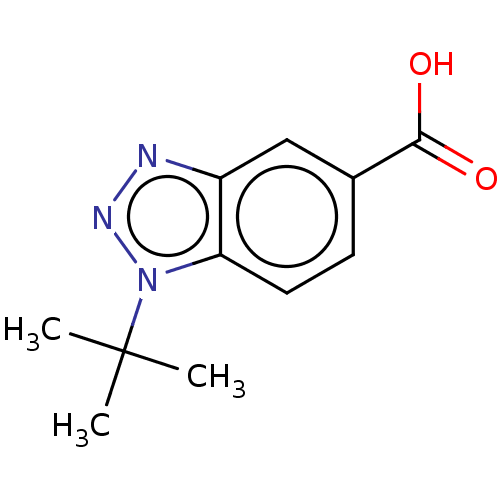
(CHEMBL372727)Show InChI InChI=1S/C11H13N3O2/c1-11(2,3)14-9-5-4-7(10(15)16)6-8(9)12-13-14/h4-6H,1-3H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475631
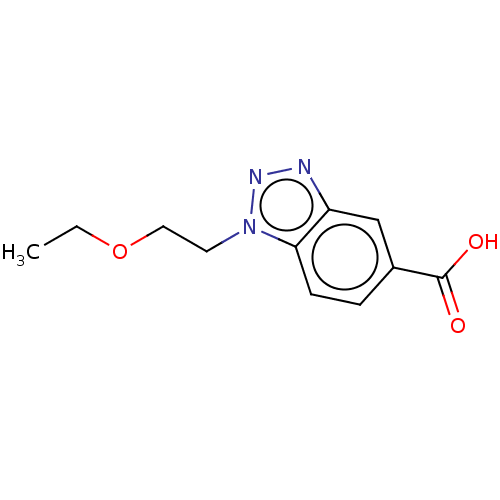
(CHEMBL202351)Show InChI InChI=1S/C11H13N3O3/c1-2-17-6-5-14-10-4-3-8(11(15)16)7-9(10)12-13-14/h3-4,7H,2,5-6H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414495

(CHEMBL562675)Show InChI InChI=1S/C14H13N3O2S2/c18-14(19)12-7-13(16-15-12)17(8-10-3-1-5-20-10)9-11-4-2-6-21-11/h1-7H,8-9H2,(H,15,16)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208140
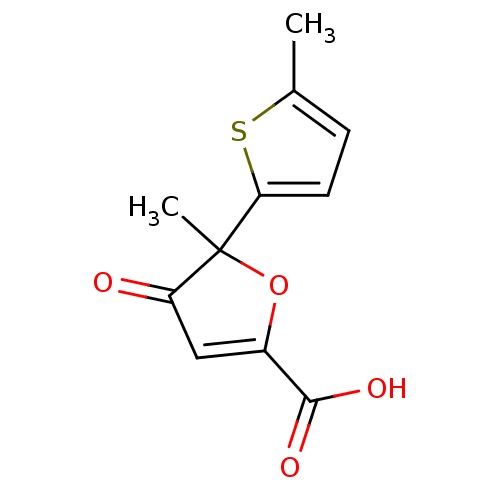
(5-methyl-5-(5-methyl-thiophen-2-yl)-4-oxo-4,5-dihy...)Show InChI InChI=1S/C11H10O4S/c1-6-3-4-9(16-6)11(2)8(12)5-7(15-11)10(13)14/h3-5H,1-2H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475629

(CHEMBL201048)Show InChI InChI=1S/C12H15N3O2/c1-2-3-4-7-15-11-6-5-9(12(16)17)8-10(11)13-14-15/h5-6,8H,2-4,7H2,1H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 288 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50337038
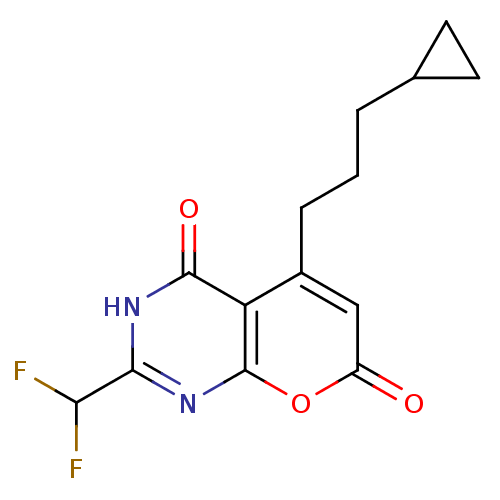
(5-(3-cyclopropylpropyl)-2-(difluoromethyl)-3H-pyra...)Show InChI InChI=1S/C14H14F2N2O3/c15-11(16)12-17-13(20)10-8(3-1-2-7-4-5-7)6-9(19)21-14(10)18-12/h6-7,11H,1-5H2,(H,17,18,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475639

(CHEMBL201409)Show InChI InChI=1S/C11H13N3O2S/c1-2-17-6-5-14-10-4-3-8(11(15)16)7-9(10)12-13-14/h3-4,7H,2,5-6H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 302 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208138
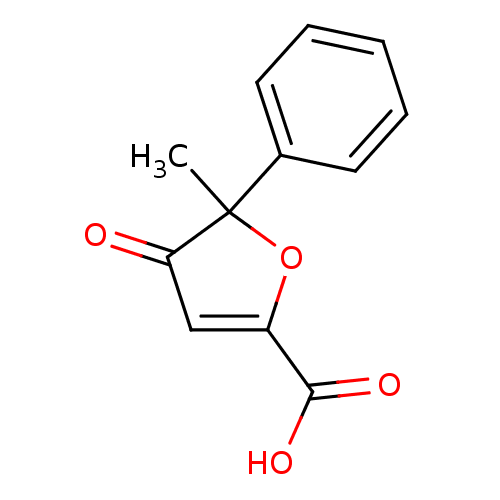
((+)-acifran | (-)-acifran | 5-Methyl-4-oxo-5-pheny...)Show InChI InChI=1S/C12H10O4/c1-12(8-5-3-2-4-6-8)10(13)7-9(16-12)11(14)15/h2-7H,1H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Agonist activity at HCA3 receptor (unknown origin) expressed in CHOK1 cells assessed as ERK1/2 phosphorylation by ELISA |
Bioorg Med Chem 23: 4013-25 (2015)
Article DOI: 10.1016/j.bmc.2015.02.018
BindingDB Entry DOI: 10.7270/Q22N558B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475645
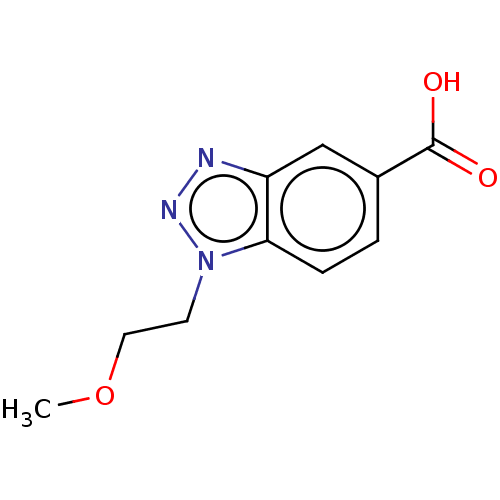
(CHEMBL202157)Show InChI InChI=1S/C10H11N3O3/c1-16-5-4-13-9-3-2-7(10(14)15)6-8(9)11-12-13/h2-3,6H,4-5H2,1H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475637
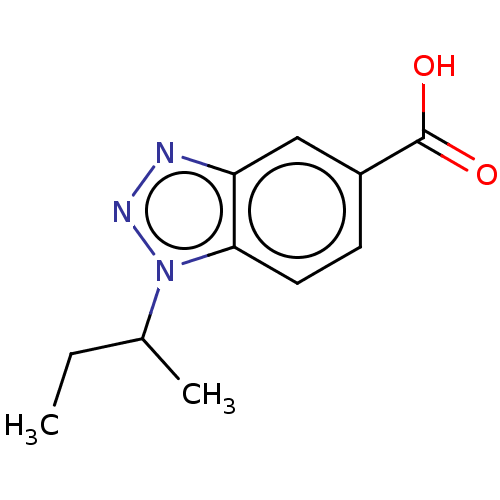
(CHEMBL202206)Show InChI InChI=1S/C11H13N3O2/c1-3-7(2)14-10-5-4-8(11(15)16)6-9(10)12-13-14/h4-7H,3H2,1-2H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 331 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50241028
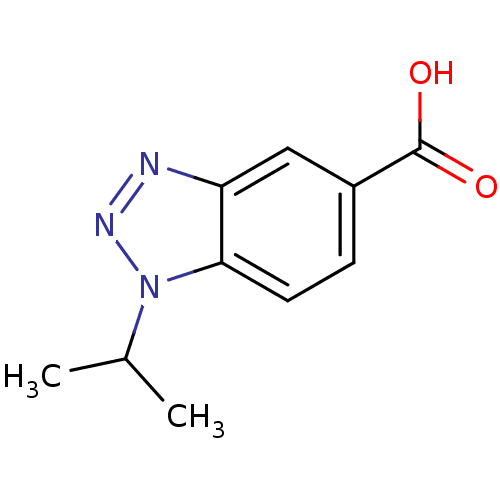
(1-Isopropyl-1H-benzotriazole-5-carboxylic acid | 1...)Show InChI InChI=1S/C10H11N3O2/c1-6(2)13-9-4-3-7(10(14)15)5-8(9)11-12-13/h3-6H,1-2H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475635
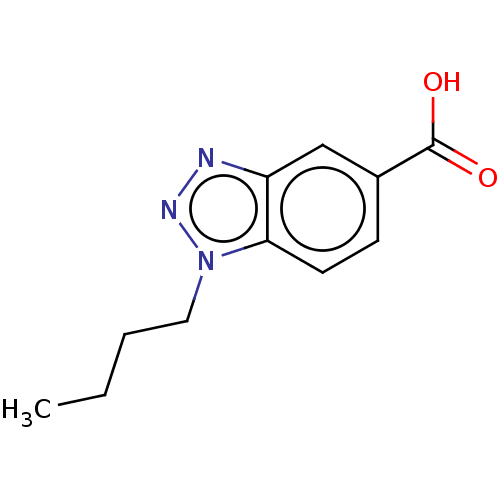
(CHEMBL202586)Show InChI InChI=1S/C11H13N3O2/c1-2-3-6-14-10-5-4-8(11(15)16)7-9(10)12-13-14/h4-5,7H,2-3,6H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 692 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50220833
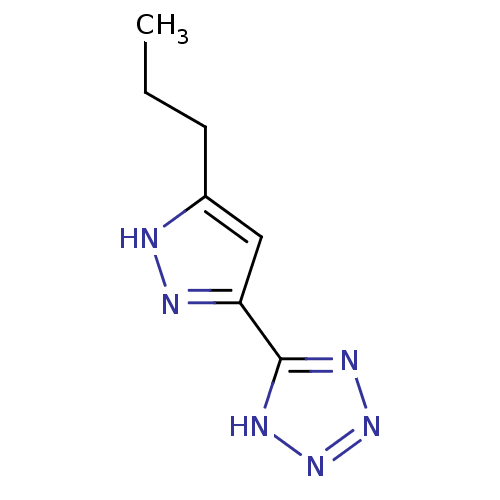
(5-(5-propyl-1H-pyrazol-3-yl)-1H-tetrazole | CHEMBL...)Show InChI InChI=1S/C7H10N6/c1-2-3-5-4-6(9-8-5)7-10-12-13-11-7/h4H,2-3H2,1H3,(H,8,9)(H,10,11,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 724 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50220833

(5-(5-propyl-1H-pyrazol-3-yl)-1H-tetrazole | CHEMBL...)Show InChI InChI=1S/C7H10N6/c1-2-3-5-4-6(9-8-5)7-10-12-13-11-7/h4H,2-3H2,1H3,(H,8,9)(H,10,11,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 724 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414499

(CHEMBL561920)Show InChI InChI=1S/C18H17N3O2/c22-18(23)16-11-17(20-19-16)21(12-14-7-3-1-4-8-14)13-15-9-5-2-6-10-15/h1-11H,12-13H2,(H,19,20)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 851 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475633
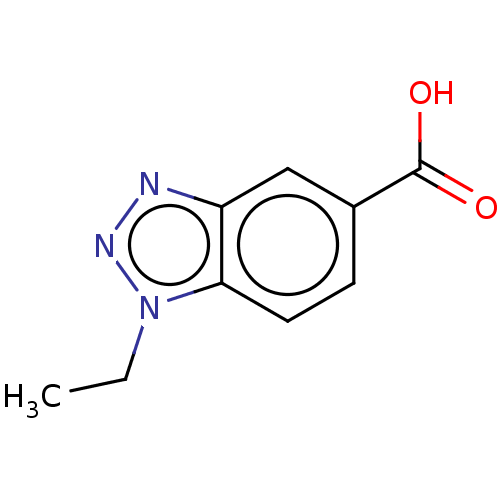
(CHEMBL440217)Show InChI InChI=1S/C9H9N3O2/c1-2-12-8-4-3-6(9(13)14)5-7(8)10-11-12/h3-5H,2H2,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 891 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475638
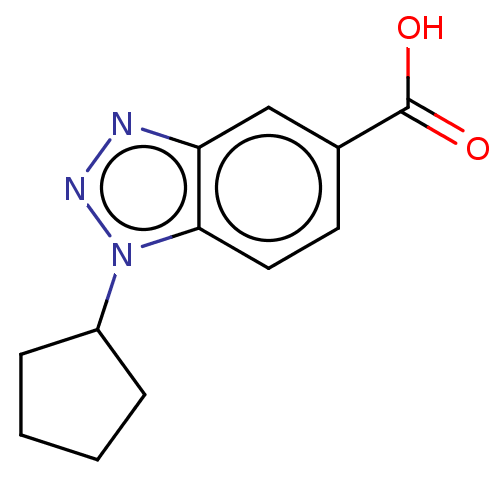
(CHEMBL201724)Show InChI InChI=1S/C12H13N3O2/c16-12(17)8-5-6-11-10(7-8)13-14-15(11)9-3-1-2-4-9/h5-7,9H,1-4H2,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50337024
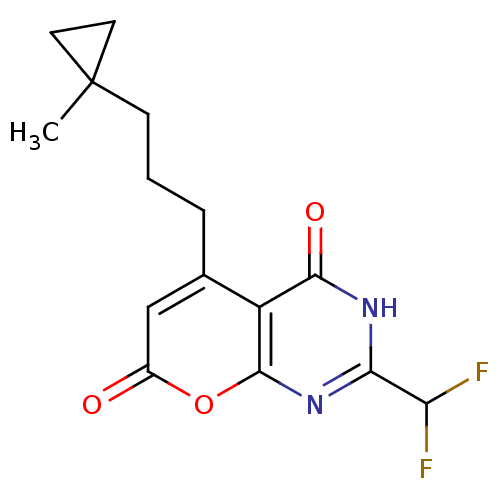
(2-(difluoromethyl)-5-(3-(1-methylcyclopropyl)propy...)Show SMILES CC1(CCCc2cc(=O)oc3nc([nH]c(=O)c23)C(F)F)CC1 Show InChI InChI=1S/C15H16F2N2O3/c1-15(5-6-15)4-2-3-8-7-9(20)22-14-10(8)13(21)18-12(19-14)11(16)17/h7,11H,2-6H2,1H3,(H,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384634

(CHEMBL2036948)Show InChI InChI=1S/C12H14N2O4/c1-6(2)3-4-7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208137
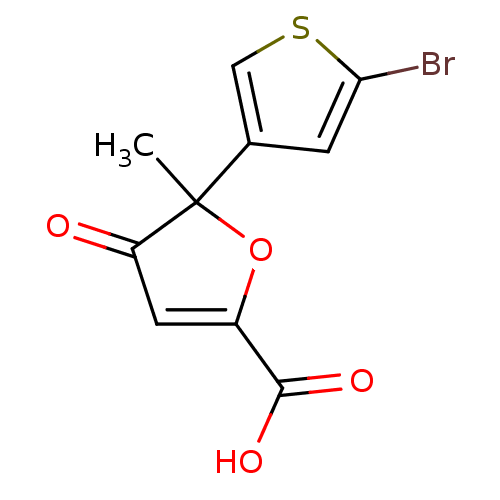
((+)-5-(5-bromo-thiophen-3-yl)-5-methyl-4-oxo-4,5-d...)Show InChI InChI=1S/C10H7BrO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109b assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208137

((+)-5-(5-bromo-thiophen-3-yl)-5-methyl-4-oxo-4,5-d...)Show InChI InChI=1S/C10H7BrO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208122
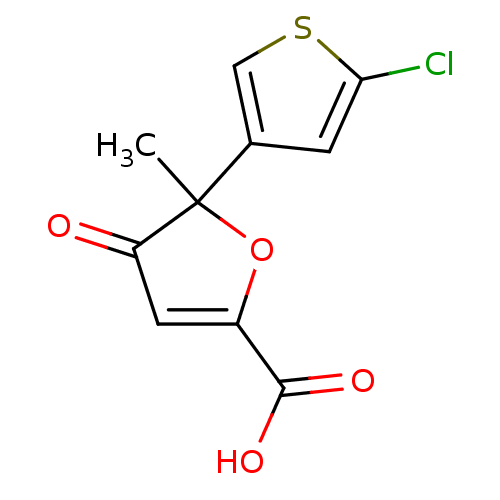
((+)-5-(5-chloro-thiophen-3-yl)-5-methyl-4-oxo-4,5-...)Show InChI InChI=1S/C10H7ClO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208122

((+)-5-(5-chloro-thiophen-3-yl)-5-methyl-4-oxo-4,5-...)Show InChI InChI=1S/C10H7ClO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109b assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208134

(5-methyl-5-(4-methyl-thiophen-2-yl)-4-oxo-4,5-dihy...)Show InChI InChI=1S/C11H10O4S/c1-6-3-9(16-5-6)11(2)8(12)4-7(15-11)10(13)14/h3-5H,1-2H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475642

(CHEMBL201513)Show InChI InChI=1S/C8H7N3O2/c1-11-7-3-2-5(8(12)13)4-6(7)9-10-11/h2-4H,1H3,(H,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475640
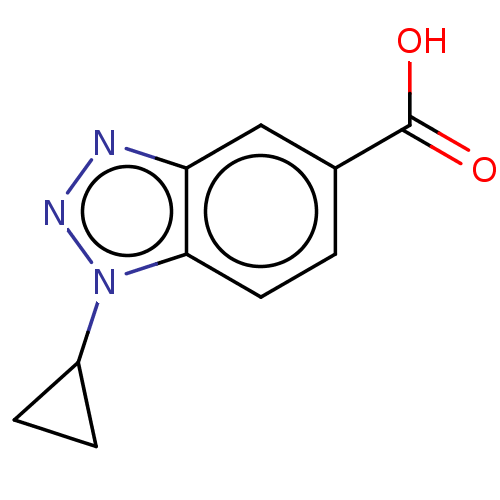
(CHEMBL380939)Show InChI InChI=1S/C10H9N3O2/c14-10(15)6-1-4-9-8(5-6)11-12-13(9)7-2-3-7/h1,4-5,7H,2-3H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475643
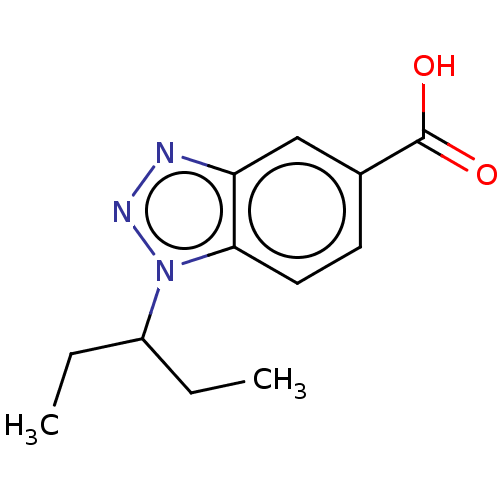
(CHEMBL370386)Show InChI InChI=1S/C12H15N3O2/c1-3-9(4-2)15-11-6-5-8(12(16)17)7-10(11)13-14-15/h5-7,9H,3-4H2,1-2H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475641

(CHEMBL440041)Show InChI InChI=1S/C11H11N3O2/c15-11(16)7-4-5-10-9(6-7)12-13-14(10)8-2-1-3-8/h4-6,8H,1-3H2,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50220853
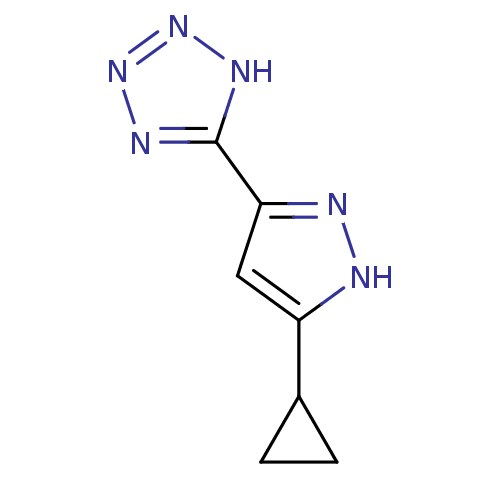
(5-(5-cyclopropyl-1H-pyrazol-3-yl)-1H-tetrazole | C...)Show InChI InChI=1S/C7H8N6/c1-2-4(1)5-3-6(9-8-5)7-10-12-13-11-7/h3-4H,1-2H2,(H,8,9)(H,10,11,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50220853

(5-(5-cyclopropyl-1H-pyrazol-3-yl)-1H-tetrazole | C...)Show InChI InChI=1S/C7H8N6/c1-2-4(1)5-3-6(9-8-5)7-10-12-13-11-7/h3-4H,1-2H2,(H,8,9)(H,10,11,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50475632

(CHEMBL382863)Show InChI InChI=1S/C11H12N2O2/c1-7(2)13-6-12-9-5-8(11(14)15)3-4-10(9)13/h3-7H,1-2H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| Assay Description
Agonistic activity at GPR109b by cAMP whole cell assay |
J Med Chem 49: 1227-30 (2006)
Article DOI: 10.1021/jm051099t
BindingDB Entry DOI: 10.7270/Q2SF2ZZ4 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208116
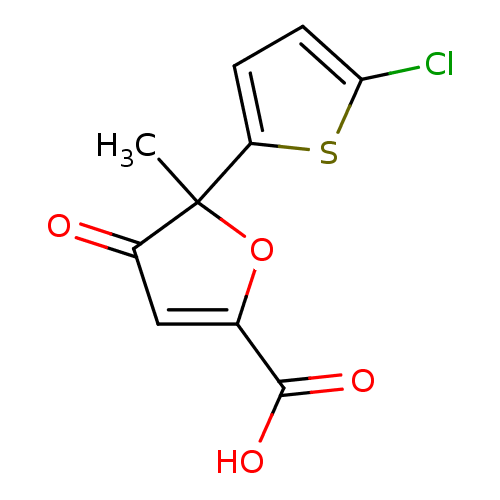
(5-(5-chloro-thiophen-2-yl)-5-methyl-4-oxo-4,5-dihy...)Show InChI InChI=1S/C10H7ClO4S/c1-10(7-2-3-8(11)16-7)6(12)4-5(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414502
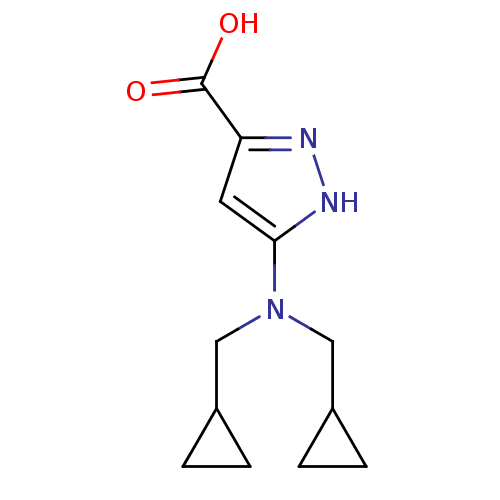
(CHEMBL561720)Show InChI InChI=1S/C12H17N3O2/c16-12(17)10-5-11(14-13-10)15(6-8-1-2-8)7-9-3-4-9/h5,8-9H,1-4,6-7H2,(H,13,14)(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208110
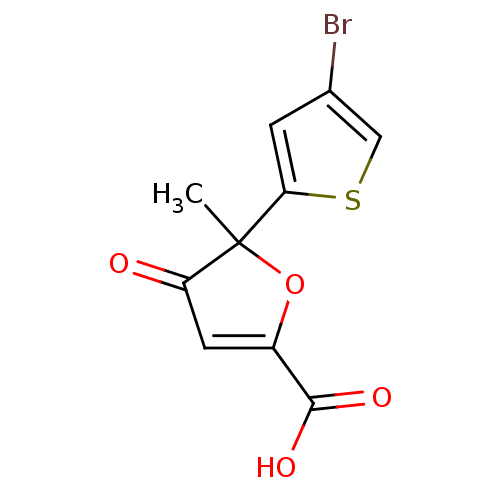
(5-methyl-5-(4-bromo-thiophen-2-yl)-4-oxo-4,5-dihyd...)Show InChI InChI=1S/C10H7BrO4S/c1-10(8-2-5(11)4-16-8)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208138

((+)-acifran | (-)-acifran | 5-Methyl-4-oxo-5-pheny...)Show InChI InChI=1S/C12H10O4/c1-12(8-5-3-2-4-6-8)10(13)7-9(16-12)11(14)15/h2-7H,1H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at N-terminal HA-tagged GPR109b receptor transfected in forskolin-stimulated cells assessed as cAMP accumulation by flashplate assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208139

(5-methyl-4-oxo-5-thiophen-3-yl-4,5-dihydro-furan-2...)Show InChI InChI=1S/C10H8O4S/c1-10(6-2-3-15-5-6)8(11)4-7(14-10)9(12)13/h2-5H,1H3,(H,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208138

((+)-acifran | (-)-acifran | 5-Methyl-4-oxo-5-pheny...)Show InChI InChI=1S/C12H10O4/c1-12(8-5-3-2-4-6-8)10(13)7-9(16-12)11(14)15/h2-7H,1H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50414506
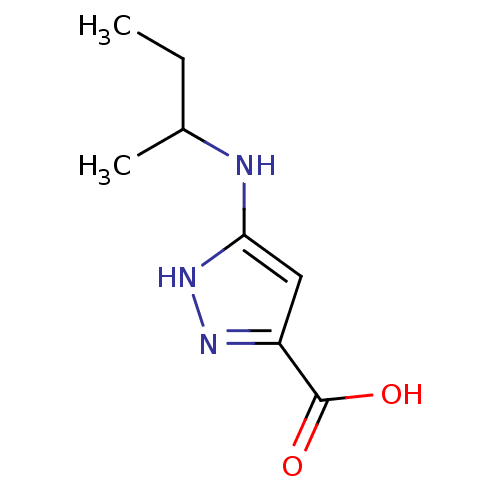
(CHEMBL553415)Show InChI InChI=1S/C8H13N3O2/c1-3-5(2)9-7-4-6(8(12)13)10-11-7/h4-5H,3H2,1-2H3,(H,12,13)(H2,9,10,11) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109b receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208125
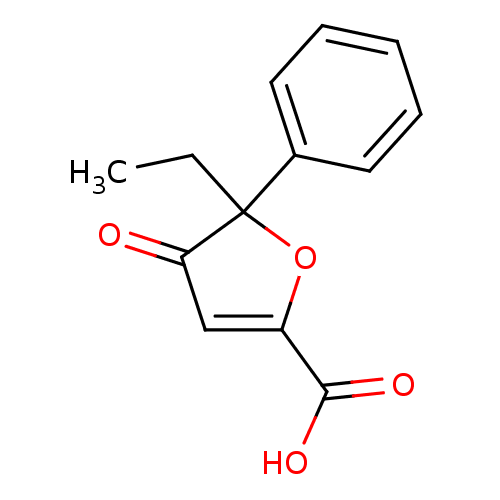
(5-ethyl-4-oxo-5-phenyl-4,5-dihydro-furan-2-carboxy...)Show InChI InChI=1S/C13H12O4/c1-2-13(9-6-4-3-5-7-9)11(14)8-10(17-13)12(15)16/h3-8H,2H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity at GPR109b in CHO cells assessed as inhibition of forskolin-induced cAMP generation |
J Med Chem 50: 1445-8 (2007)
Article DOI: 10.1021/jm070022x
BindingDB Entry DOI: 10.7270/Q2BK1C1Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data