 Found 284 hits of ki for UniProtKB: P31390
Found 284 hits of ki for UniProtKB: P31390 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(RAT) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of [3H]7-OH-DPAT binding to Histamine H1 receptor in rat tissue homogenate |
J Med Chem 45: 344-59 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3G26 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50115644
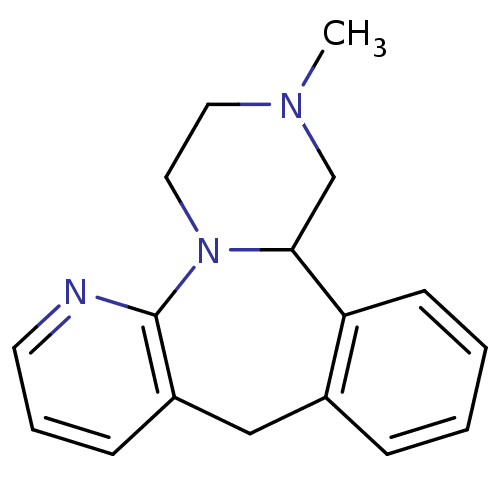
((+/-)-12-Methyl-1,2,3,4,9,13b-hexahydro-2,4a,5-tri...)Show InChI InChI=1S/C17H19N3/c1-19-9-10-20-16(12-19)15-7-3-2-5-13(15)11-14-6-4-8-18-17(14)20/h2-8,16H,9-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon International
Curated by PDSP Ki Database
| |
Neuropharmacology 27: 399-408 (1988)
Article DOI: 10.1016/0028-3908(88)90149-9
BindingDB Entry DOI: 10.7270/Q2B27SSW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50115644

((+/-)-12-Methyl-1,2,3,4,9,13b-hexahydro-2,4a,5-tri...)Show InChI InChI=1S/C17H19N3/c1-19-9-10-20-16(12-19)15-7-3-2-5-13(15)11-14-6-4-8-18-17(14)20/h2-8,16H,9-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon International
Curated by PDSP Ki Database
| |
Neuropharmacology 27: 399-408 (1988)
Article DOI: 10.1016/0028-3908(88)90149-9
BindingDB Entry DOI: 10.7270/Q2B27SSW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50449566
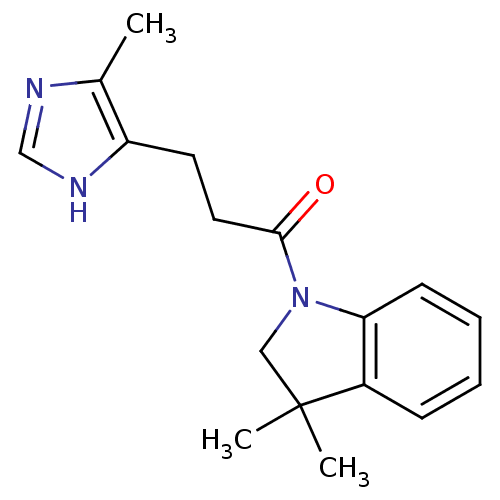
(CHEMBL414174)Show InChI InChI=1S/C17H21N3O/c1-12-14(19-11-18-12)8-9-16(21)20-10-17(2,3)13-6-4-5-7-15(13)20/h4-7,11H,8-10H2,1-3H3,(H,18,19) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested for antagonistic activity on 5-hydroxytryptamine 3 receptor mediated effects of 5-HT in guinea pig isolated ileum |
Citation and Details
Article DOI: 10.1016/S0960-894X(00)80418-7
BindingDB Entry DOI: 10.7270/Q2MG7R17 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50115644

((+/-)-12-Methyl-1,2,3,4,9,13b-hexahydro-2,4a,5-tri...)Show InChI InChI=1S/C17H19N3/c1-19-9-10-20-16(12-19)15-7-3-2-5-13(15)11-14-6-4-8-18-17(14)20/h2-8,16H,9-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon International
Curated by PDSP Ki Database
| |
Neuropharmacology 27: 399-408 (1988)
Article DOI: 10.1016/0028-3908(88)90149-9
BindingDB Entry DOI: 10.7270/Q2B27SSW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50079527
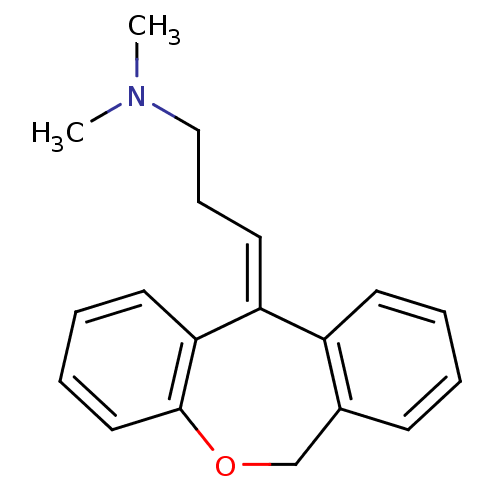
((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35256
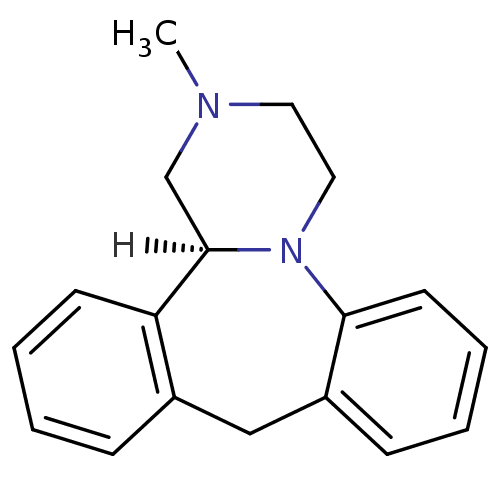
((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon International
Curated by PDSP Ki Database
| |
Neuropharmacology 27: 399-408 (1988)
Article DOI: 10.1016/0028-3908(88)90149-9
BindingDB Entry DOI: 10.7270/Q2B27SSW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50025124
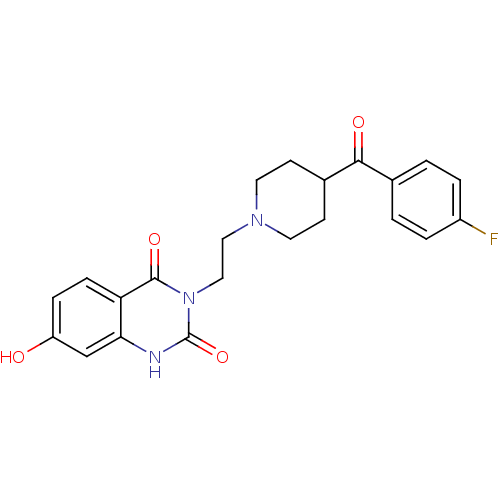
(3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-...)Show SMILES Oc1ccc2c(c1)[nH]c(=O)n(CCN1CCC(CC1)C(=O)c1ccc(F)cc1)c2=O Show InChI InChI=1S/C22H22FN3O4/c23-16-3-1-14(2-4-16)20(28)15-7-9-25(10-8-15)11-12-26-21(29)18-6-5-17(27)13-19(18)24-22(26)30/h1-6,13,15,27H,7-12H2,(H,24,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding activity against [3H]pyrilamine as radioligand for Histamine H1 receptor |
J Med Chem 29: 1663-8 (1986)
BindingDB Entry DOI: 10.7270/Q2RX9B3N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50062261
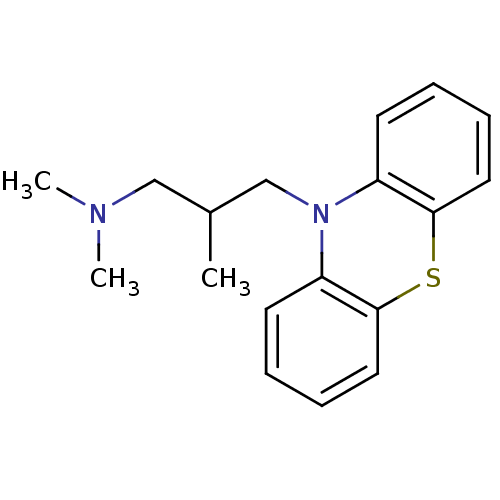
(CHEMBL829 | Dimethyl-(2-methyl-3-phenothiazin-10-y...)Show InChI InChI=1S/C18H22N2S/c1-14(12-19(2)3)13-20-15-8-4-6-10-17(15)21-18-11-7-5-9-16(18)20/h4-11,14H,12-13H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM81472

(CAS_10646 | NSC_10646 | Pyrathiazine)Show InChI InChI=1S/C18H20N2S/c1-3-9-17-15(7-1)20(14-13-19-11-5-6-12-19)16-8-2-4-10-18(16)21-17/h1-4,7-10H,5-6,11-14H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35256

((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon International
Curated by PDSP Ki Database
| |
Neuropharmacology 27: 399-408 (1988)
Article DOI: 10.1016/0028-3908(88)90149-9
BindingDB Entry DOI: 10.7270/Q2B27SSW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM84994
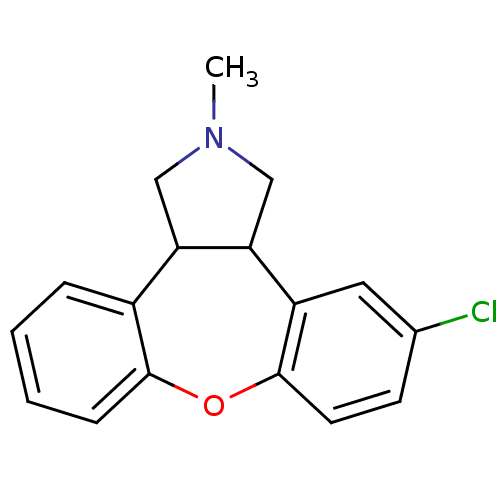
(CAS_163091 | NSC_163091 | ORG-5222)Show InChI InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17)15(14)10-19/h2-8,14-15H,9-10H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 14: 87-96 (1996)
Article DOI: 10.1016/0893-133X(94)00129-N
BindingDB Entry DOI: 10.7270/Q2445K1M |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35938
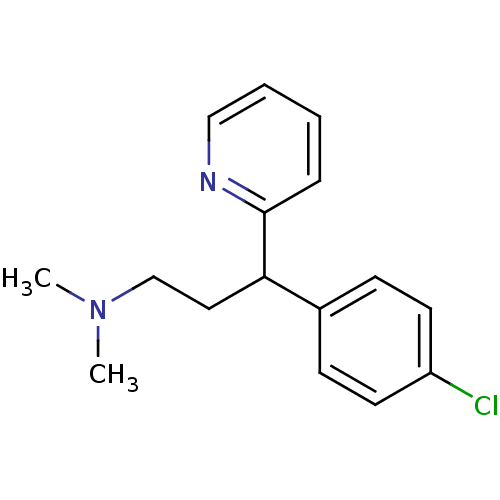
(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-Pyrilamine from histamine H1 receptor in male SpragueDawley rat brain membranes |
Bioorg Med Chem Lett 13: 1959-61 (2003)
BindingDB Entry DOI: 10.7270/Q27P8XS6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50010859
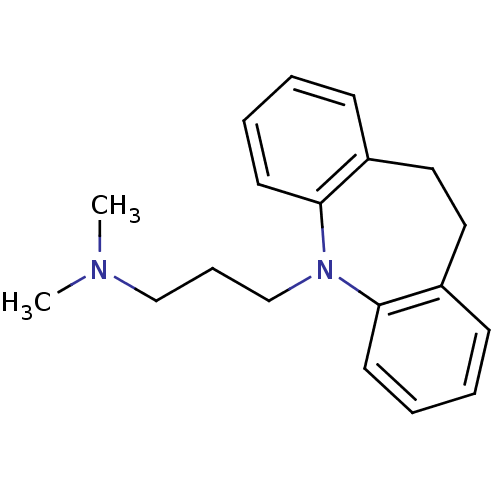
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Industri A/S
Curated by PDSP Ki Database
| |
Eur J Pharmacol 166: 493-504 (1989)
Article DOI: 10.1016/0014-2999(89)90363-4
BindingDB Entry DOI: 10.7270/Q28C9TR3 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM81464
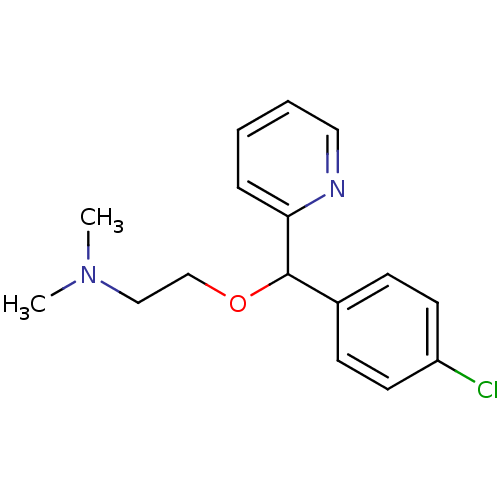
(2-[(4-chlorophenyl)-(2-pyridyl)methoxy]ethyl-dimet...)Show InChI InChI=1S/C16H19ClN2O/c1-19(2)11-12-20-16(15-5-3-4-10-18-15)13-6-8-14(17)9-7-13/h3-10,16H,11-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM81470

(CAS_14677 | Methdilazine | NSC_14677 | US9504692, ...)Show InChI InChI=1S/C18H20N2S/c1-19-11-10-14(12-19)13-20-15-6-2-4-8-17(15)21-18-9-5-3-7-16(18)20/h2-9,14H,10-13H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50137980
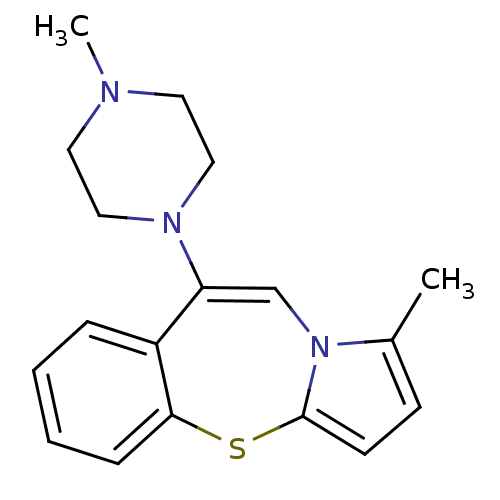
(1-Methyl-9-(4-methyl-piperazin-1-yl)-4-thia-10a-az...)Show InChI InChI=1S/C18H21N3S/c1-14-7-8-18-21(14)13-16(20-11-9-19(2)10-12-20)15-5-3-4-6-17(15)22-18/h3-8,13H,9-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Histamine H1 receptor of rat frontal cortex homogenate by using radioligand [3H]pyrilamine |
J Med Chem 47: 143-57 (2003)
Article DOI: 10.1021/jm0309811
BindingDB Entry DOI: 10.7270/Q2VM4CRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50137976
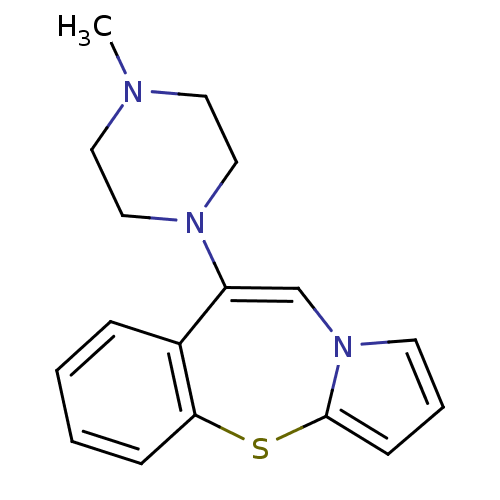
(9-(4-Methyl-piperazin-1-yl)-4-thia-10a-aza-benzo[f...)Show InChI InChI=1S/C17H19N3S/c1-18-9-11-19(12-10-18)15-13-20-8-4-7-17(20)21-16-6-3-2-5-14(15)16/h2-8,13H,9-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Histamine H1 receptor of rat frontal cortex homogenate by using radioligand [3H]pyrilamine |
J Med Chem 47: 143-57 (2003)
Article DOI: 10.1021/jm0309811
BindingDB Entry DOI: 10.7270/Q2VM4CRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50017696
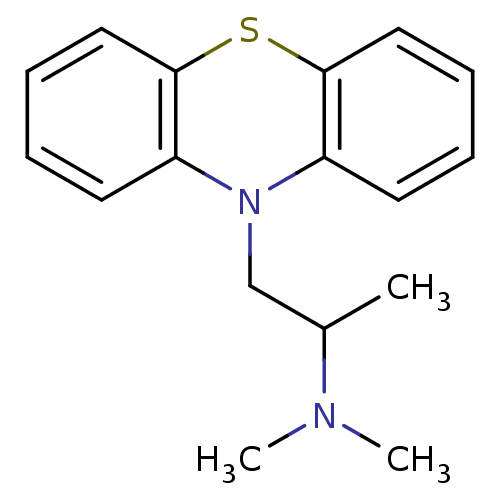
((2-dimethylamino-2-methyl)ethyl-N-dibenzoparathiaz...)Show InChI InChI=1S/C17H20N2S/c1-13(18(2)3)12-19-14-8-4-6-10-16(14)20-17-11-7-5-9-15(17)19/h4-11,13H,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Histamine H1 receptor of rat frontal cortex homogenate by using radioligand [3H]pyrilamine |
J Med Chem 47: 143-57 (2003)
Article DOI: 10.1021/jm0309811
BindingDB Entry DOI: 10.7270/Q2VM4CRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50111631
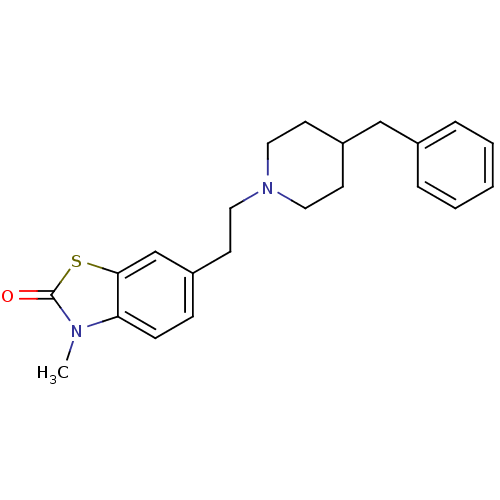
(6-[2-(4-Benzyl-piperidin-1-yl)-ethyl]-3-methyl-3H-...)Show InChI InChI=1S/C22H26N2OS/c1-23-20-8-7-18(16-21(20)26-22(23)25)9-12-24-13-10-19(11-14-24)15-17-5-3-2-4-6-17/h2-8,16,19H,9-15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Displacement of [3H]- pyrilamine from rat brain membrane Histamine H1 receptor |
Bioorg Med Chem Lett 12: 1149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PM6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50241333

(4-(benzhydryloxy)-1-methylpiperidine | CHEMBL1492 ...)Show InChI InChI=1S/C19H23NO/c1-20-14-12-18(13-15-20)21-19(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-19H,12-15H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM22868
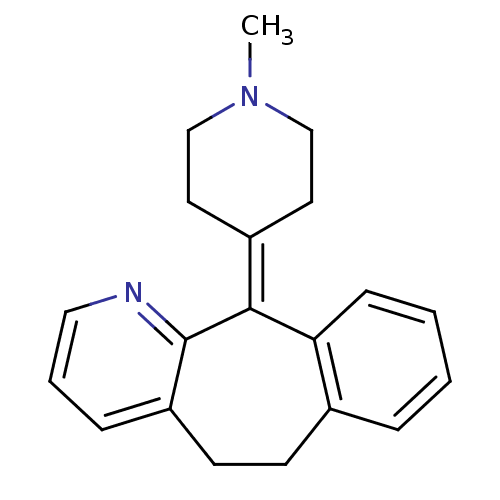
(11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-b...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C20H22N2/c1-22-13-10-16(11-14-22)19-18-7-3-2-5-15(18)8-9-17-6-4-12-21-20(17)19/h2-7,12H,8-11,13-14H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor in rat brain membranes was evaluated using [3H]-pyrilamine as radioligand |
J Med Chem 34: 457-61 (1991)
BindingDB Entry DOI: 10.7270/Q2BV7FK0 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM82548

(CAS_897-15-4 | Dothiepin | NSC_3155)Show InChI InChI=1S/C19H21NS/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Cell Mol Neurobiol 19: 467-89 (1999)
Article DOI: 10.1023/a:1006986824213
BindingDB Entry DOI: 10.7270/Q2WQ02CP |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50017696

((2-dimethylamino-2-methyl)ethyl-N-dibenzoparathiaz...)Show InChI InChI=1S/C17H20N2S/c1-13(18(2)3)12-19-14-8-4-6-10-16(14)20-17-11-7-5-9-15(17)19/h4-11,13H,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Italia
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 352: 276-82 (1995)
Article DOI: 10.1007/bf00168557
BindingDB Entry DOI: 10.7270/Q2Q52N48 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35256

((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon International
Curated by PDSP Ki Database
| |
Neuropharmacology 27: 399-408 (1988)
Article DOI: 10.1016/0028-3908(88)90149-9
BindingDB Entry DOI: 10.7270/Q2B27SSW |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50020712
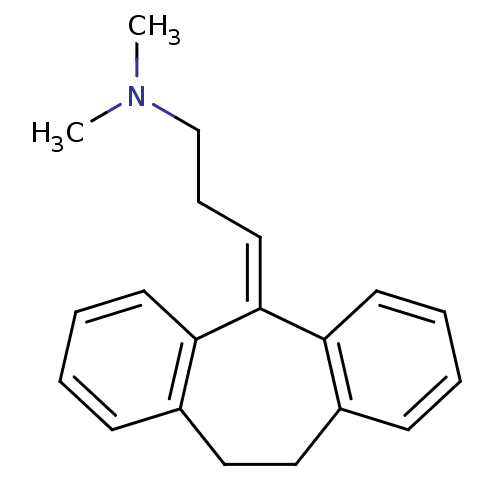
(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Histamine H1 receptor of rat frontal cortex homogenate by using radioligand [3H]pyrilamine |
J Med Chem 47: 143-57 (2003)
Article DOI: 10.1021/jm0309811
BindingDB Entry DOI: 10.7270/Q2VM4CRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50108592
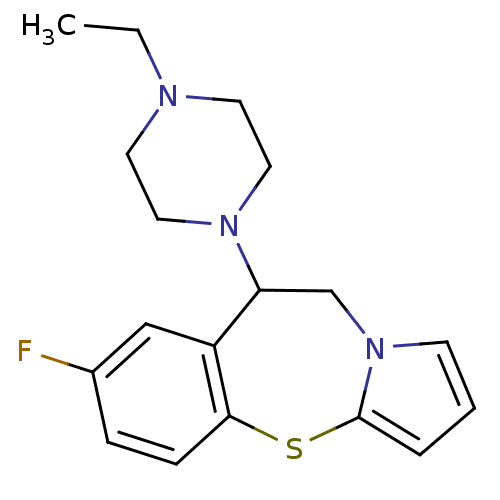
((+/-)9-(4-Ethyl-piperazin-1-yl)-7-fluoro-9,10-dihy...)Show InChI InChI=1S/C18H22FN3S/c1-2-20-8-10-21(11-9-20)16-13-22-7-3-4-18(22)23-17-6-5-14(19)12-15(16)17/h3-7,12,16H,2,8-11,13H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of [3H]pyrilamine binding to Histamine H1 receptor in rat frontal cortex homogenate |
J Med Chem 45: 344-59 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3G26 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM81469

(CAS_91-80-5 | Methapyrilene | NSC_4098)Show InChI InChI=1S/C14H19N3S/c1-16(2)9-10-17(12-13-6-5-11-18-13)14-7-3-4-8-15-14/h3-8,11H,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50409601
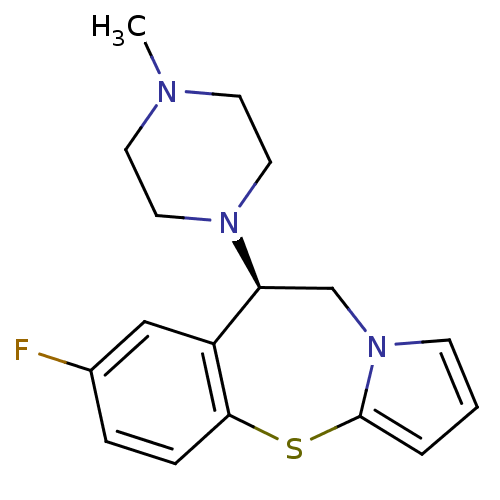
(CHEMBL2111783)Show InChI InChI=1S/C17H20FN3S/c1-19-7-9-20(10-8-19)15-12-21-6-2-3-17(21)22-16-5-4-13(18)11-14(15)16/h2-6,11,15H,7-10,12H2,1H3/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of [3H]pyrilamine binding to Histamine H1 receptor in rat frontal cortex homogenate |
J Med Chem 45: 344-59 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3G26 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330747
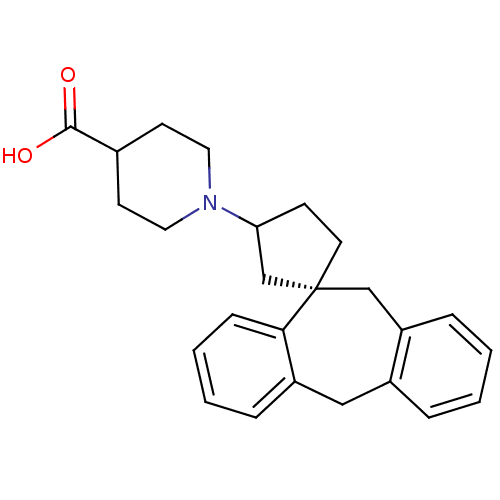
((-)-1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'...)Show SMILES OC(=O)C1CCN(CC1)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C25H29NO2/c27-24(28)18-10-13-26(14-11-18)22-9-12-25(17-22)16-21-7-2-1-5-19(21)15-20-6-3-4-8-23(20)25/h1-8,18,22H,9-17H2,(H,27,28)/t22?,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50017666
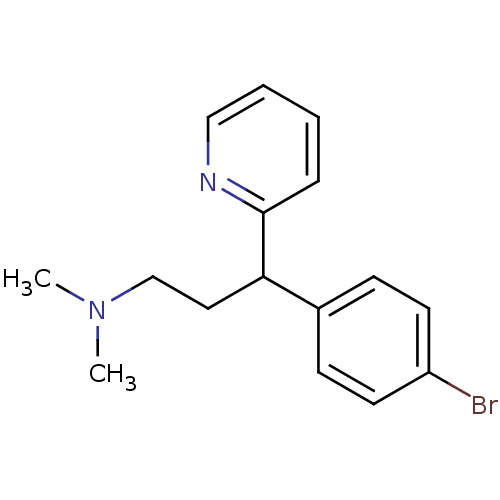
(1-(p-bromophenyl)-1-(2-pyridyl)-3-dimethylaminopro...)Show InChI InChI=1S/C16H19BrN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against rat cortical H1 receptor by radioligand [3H]-pyrilamine binding assay. |
J Med Chem 39: 2764-72 (1996)
Article DOI: 10.1021/jm9601720
BindingDB Entry DOI: 10.7270/Q2SB44VK |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330752
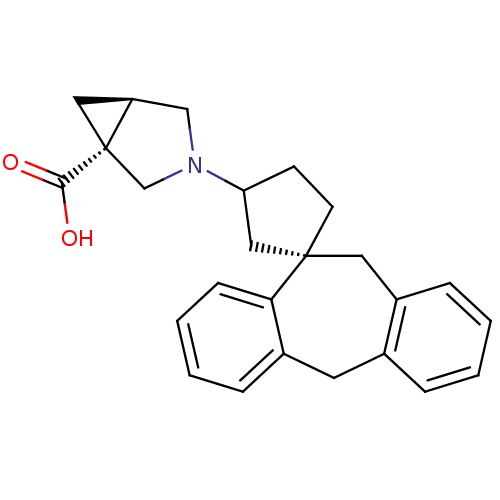
(3-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...)Show SMILES OC(=O)[C@]12C[C@H]1CN(C2)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C25H27NO2/c27-23(28)25-13-20(25)15-26(16-25)21-9-10-24(14-21)12-19-7-2-1-5-17(19)11-18-6-3-4-8-22(18)24/h1-8,20-21H,9-16H2,(H,27,28)/t20-,21?,24-,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35938

(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes |
Bioorg Med Chem Lett 8: 3469-74 (1999)
BindingDB Entry DOI: 10.7270/Q21J98X8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM35938

(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor in rat brain membranes was evaluated using [3H]-pyrilamine as radioligand |
J Med Chem 34: 457-61 (1991)
BindingDB Entry DOI: 10.7270/Q2BV7FK0 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM82479
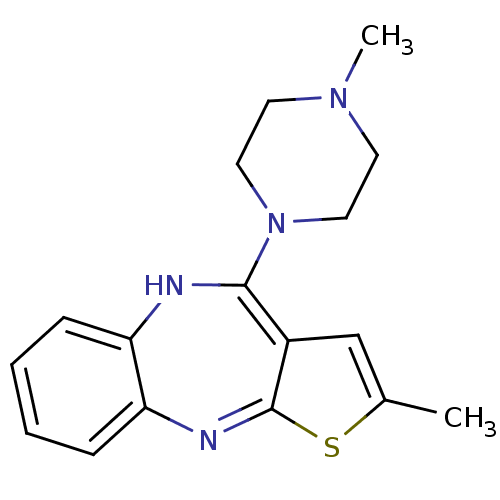
(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 18: 63-101 (1998)
Article DOI: 10.1016/S0893-133X(97)00112-7
BindingDB Entry DOI: 10.7270/Q2SF2TQB |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50292411
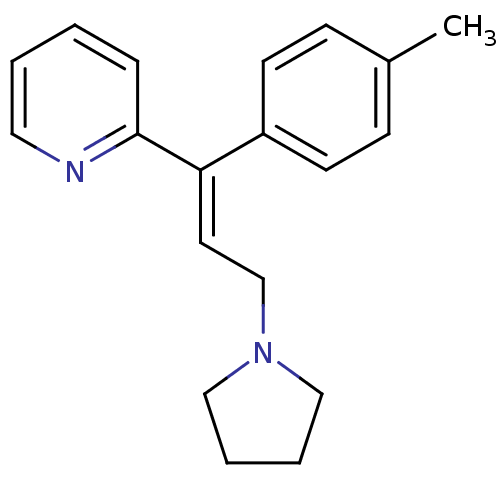
((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 75: 6290-4 (1978)
Article DOI: 10.1073/pnas.75.12.6290
BindingDB Entry DOI: 10.7270/Q2FN14P6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330751
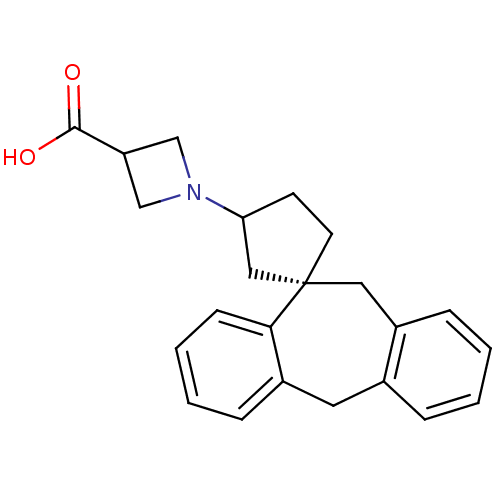
(1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...)Show SMILES OC(=O)C1CN(C1)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C23H25NO2/c25-22(26)19-14-24(15-19)20-9-10-23(13-20)12-18-7-2-1-5-16(18)11-17-6-3-4-8-21(17)23/h1-8,19-20H,9-15H2,(H,25,26)/t20?,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 14: 87-96 (1996)
Article DOI: 10.1016/0893-133X(94)00129-N
BindingDB Entry DOI: 10.7270/Q2445K1M |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50128888

(CHEMBL58973 | [3-(4-Chloro-phenyl)-3-pyridin-2-yl-...)Show SMILES CN(CCCCCc1cnc[nH]1)CCC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C23H29ClN4/c1-28(15-6-2-3-7-21-17-25-18-27-21)16-13-22(23-8-4-5-14-26-23)19-9-11-20(24)12-10-19/h4-5,8-12,14,17-18,22H,2-3,6-7,13,15-16H2,1H3,(H,25,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-Pyrilamine from histamine H1 receptor in male SpragueDawley rat brain membranes |
Bioorg Med Chem Lett 13: 1959-61 (2003)
BindingDB Entry DOI: 10.7270/Q27P8XS6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50063449
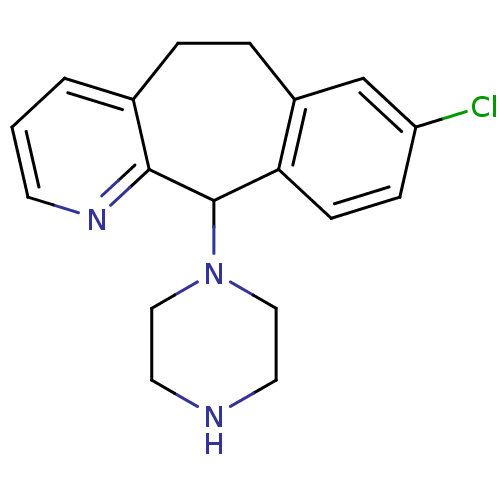
(8-Chloro-11-piperazin-1-yl-6,11-dihydro-5H-benzo[5...)Show InChI InChI=1S/C18H20ClN3/c19-15-5-6-16-14(12-15)4-3-13-2-1-7-21-17(13)18(16)22-10-8-20-9-11-22/h1-2,5-7,12,18,20H,3-4,8-11H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes |
Bioorg Med Chem Lett 8: 3469-74 (1999)
BindingDB Entry DOI: 10.7270/Q21J98X8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50073184
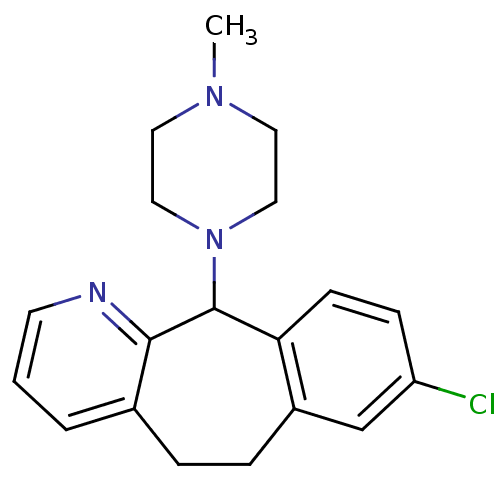
(8-Chloro-11-(4-methyl-piperazin-1-yl)-6,11-dihydro...)Show InChI InChI=1S/C19H22ClN3/c1-22-9-11-23(12-10-22)19-17-7-6-16(20)13-15(17)5-4-14-3-2-8-21-18(14)19/h2-3,6-8,13,19H,4-5,9-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes |
Bioorg Med Chem Lett 8: 3469-74 (1999)
BindingDB Entry DOI: 10.7270/Q21J98X8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 14: 87-96 (1996)
Article DOI: 10.1016/0893-133X(94)00129-N
BindingDB Entry DOI: 10.7270/Q2445K1M |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50111627
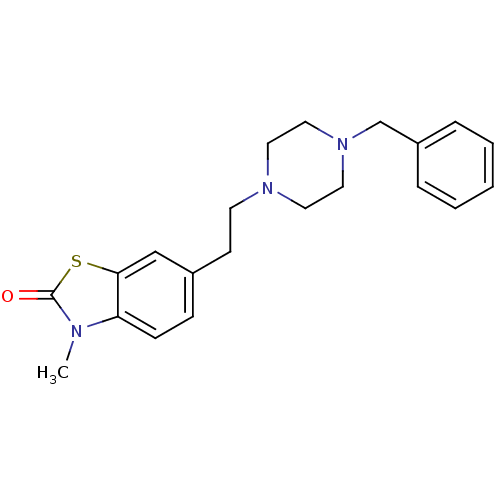
(6-[2-(4-Benzyl-piperazin-1-yl)-ethyl]-3-methyl-3H-...)Show InChI InChI=1S/C21H25N3OS/c1-22-19-8-7-17(15-20(19)26-21(22)25)9-10-23-11-13-24(14-12-23)16-18-5-3-2-4-6-18/h2-8,15H,9-14,16H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
Displacement of [3H]- pyrilamine from rat brain membrane Histamine H1 receptor |
Bioorg Med Chem Lett 12: 1149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PM6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50128875
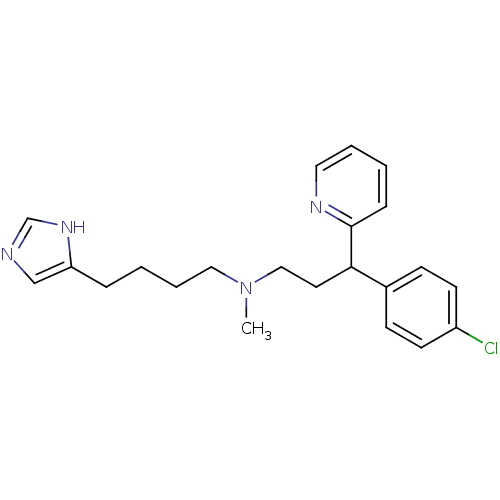
(CHEMBL292195 | [3-(4-Chloro-phenyl)-3-pyridin-2-yl...)Show SMILES CN(CCCCc1cnc[nH]1)CCC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C22H27ClN4/c1-27(14-5-3-6-20-16-24-17-26-20)15-12-21(22-7-2-4-13-25-22)18-8-10-19(23)11-9-18/h2,4,7-11,13,16-17,21H,3,5-6,12,14-15H2,1H3,(H,24,26) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-Pyrilamine from histamine H1 receptor in male SpragueDawley rat brain membranes |
Bioorg Med Chem Lett 13: 1959-61 (2003)
BindingDB Entry DOI: 10.7270/Q27P8XS6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data