

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007830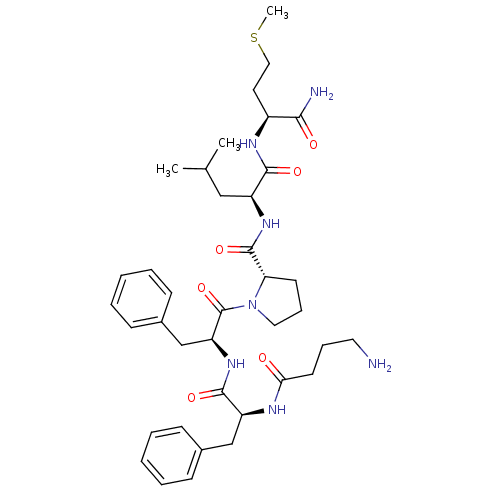 (1-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propionyla...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007827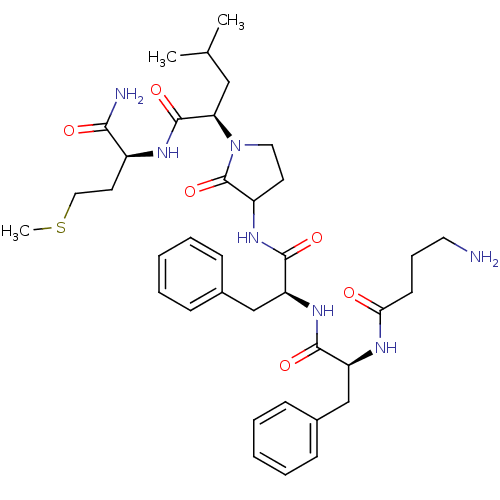 (2-(3-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001447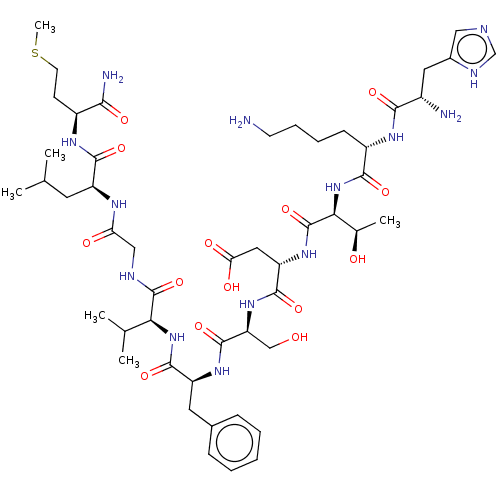 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a |
UMR UdS/CNRS 7200 Curated by ChEMBL | Assay Description Allosteric modulation of rat NK2 receptor expressed in HEK293 cells assessed as increase in intracellular calcium level by spectrofluorimetry | J Med Chem 52: 5999-6011 (2009) Article DOI: 10.1021/jm900671k BindingDB Entry DOI: 10.7270/Q2K0757R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007826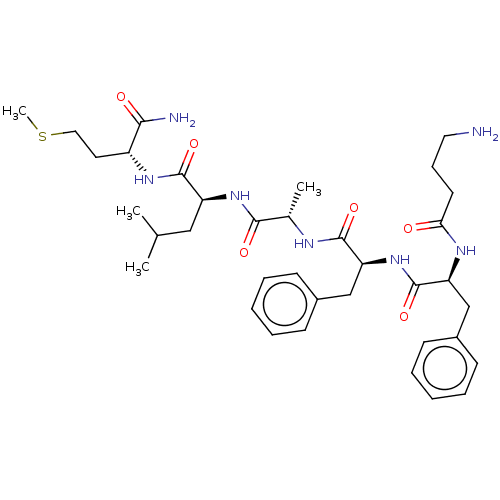 (2-(2-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001596 (3-(2-Acetylamino-6-amino-hexanoylamino)-N-{1-[1-(1...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001447 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007825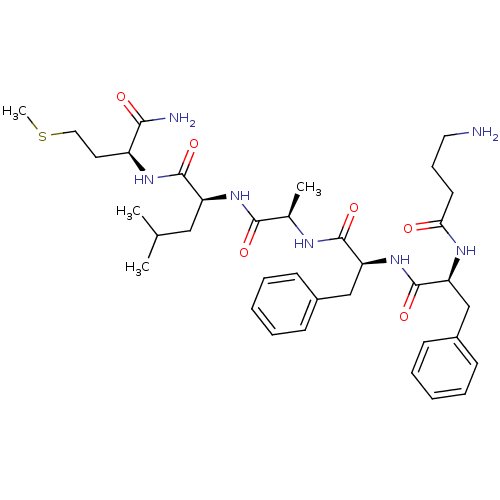 (2-(2-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001594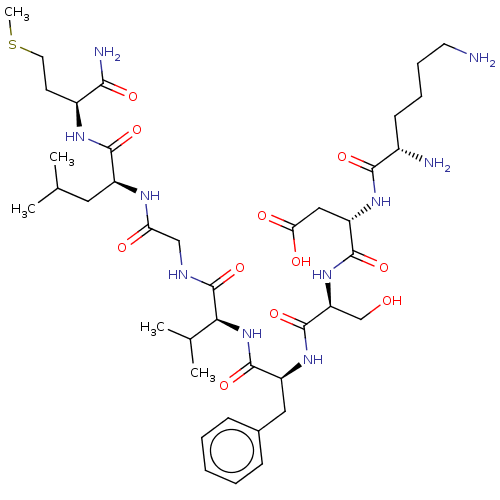 (CHEMBL335054 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001593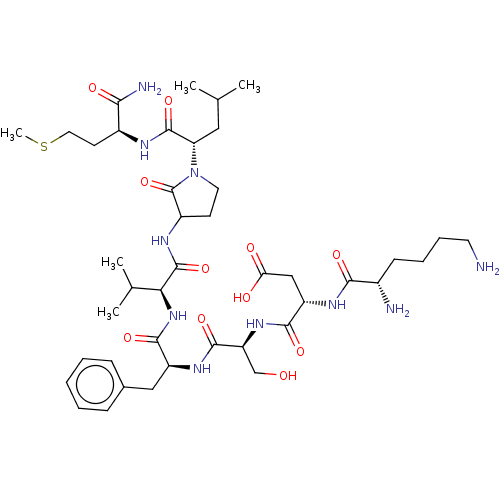 (CHEMBL131872 | N-{1-[1-(1-{1-[1-(1-Carbamoyl-3-met...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007831 (2-(2-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007829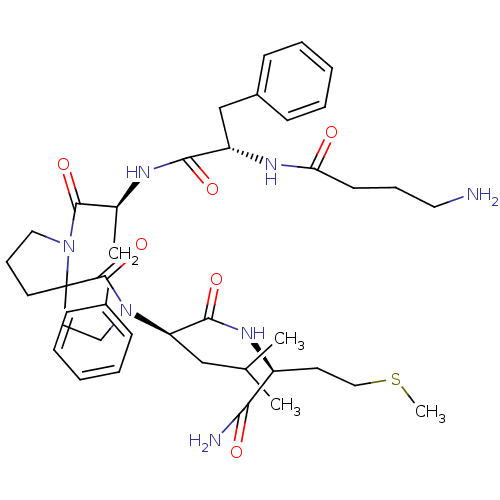 (2-(1-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001599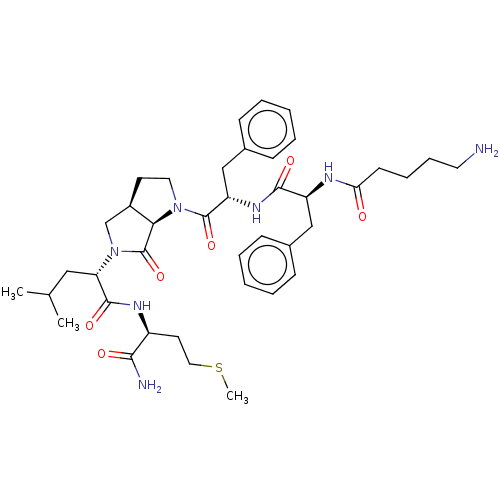 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50079412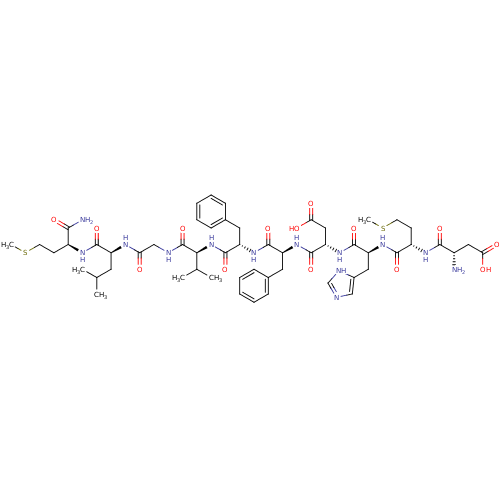 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001597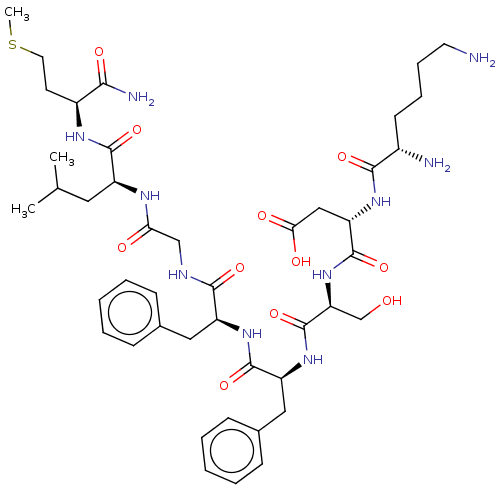 (CHEMBL134481 | N-(1-{1-[1-({[1-(1-Carbamoyl-3-meth...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001600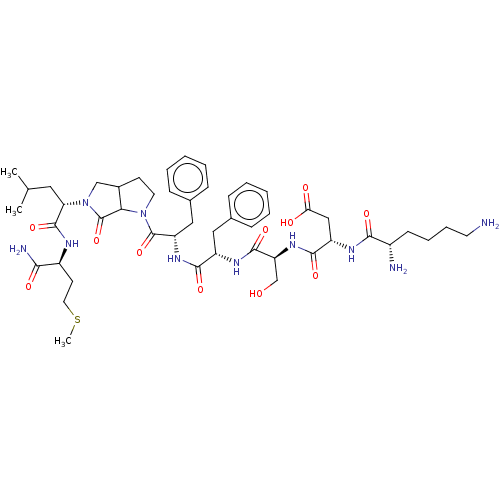 (CHEMBL267712 | N-{1-[1-(1-Benzyl-2-{5-[1-(1-carbam...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001592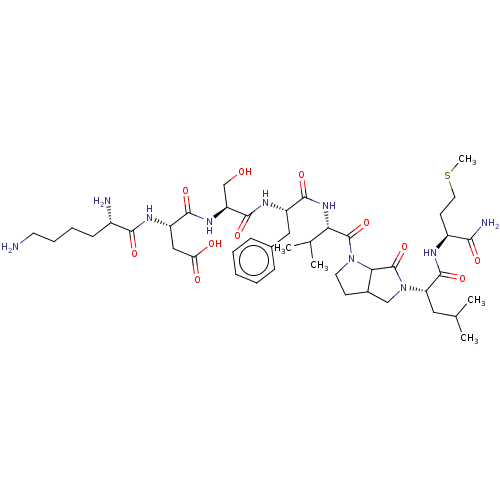 (CHEMBL131923 | N-{1-[1-(1-{5-[1-(1-Carbamoyl-3-met...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007832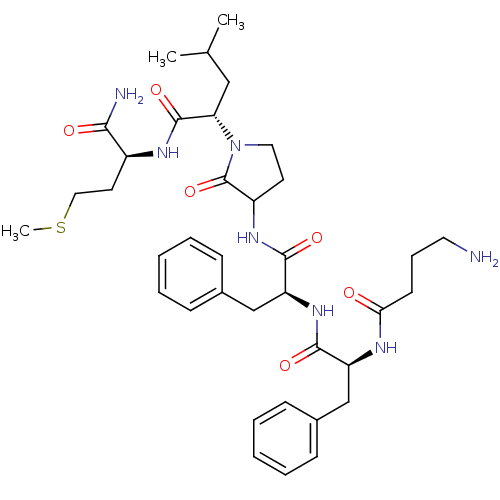 (2-(3-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001601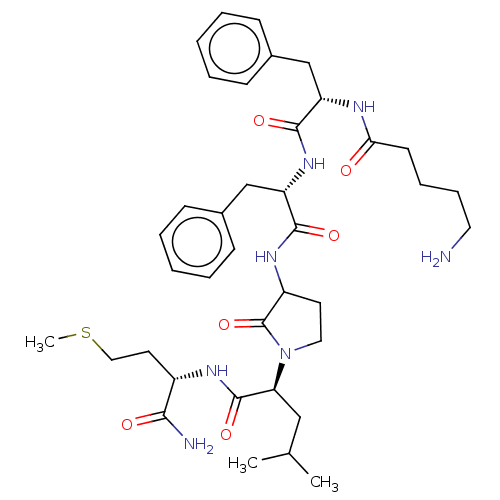 (2-(3-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001447 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 64.8 | n/a | n/a | n/a | n/a |
UMR UdS/CNRS 7200 Curated by ChEMBL | Assay Description Allosteric modulation of rat NK2 receptor expressed in HEK293 cells assessed as cAMP production by radioimmunoassay | J Med Chem 52: 5999-6011 (2009) Article DOI: 10.1021/jm900671k BindingDB Entry DOI: 10.7270/Q2K0757R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001607 (1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propiony...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001606 (2-(2-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007822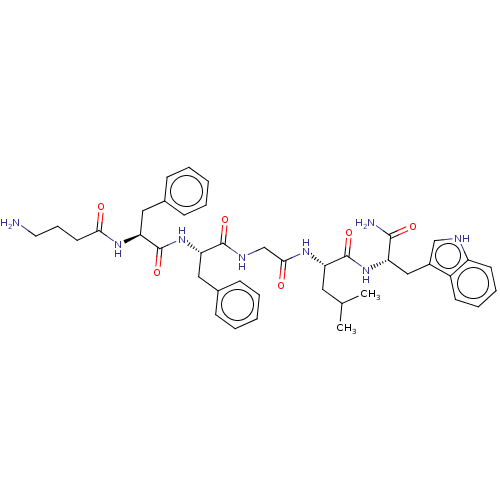 (2-(2-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >100 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonistic activity at neurokinin-1 (NK-1) receptor in guinea pig ileum longitudinal smooth muscle | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007822 (2-(2-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >100 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity was measured at neurokinin-2 receptor in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007823 (1-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propionyla...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 188 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007824 (2-(2-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >300 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50007828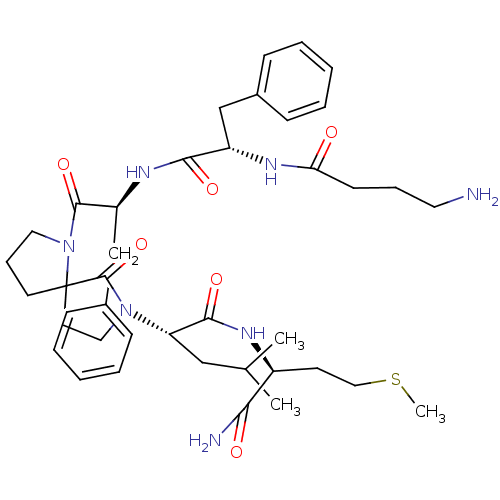 (2-(1-{2-[2-(4-Amino-butyrylamino)-3-phenyl-propion...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >500 | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Agonist activity at tachykinin receptor 2 in the rat colon muscularis mucosae | J Med Chem 33: 1848-51 (1990) BindingDB Entry DOI: 10.7270/Q2668C5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001602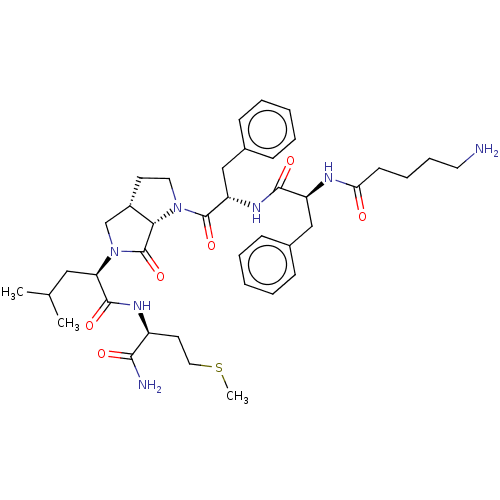 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 566 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001605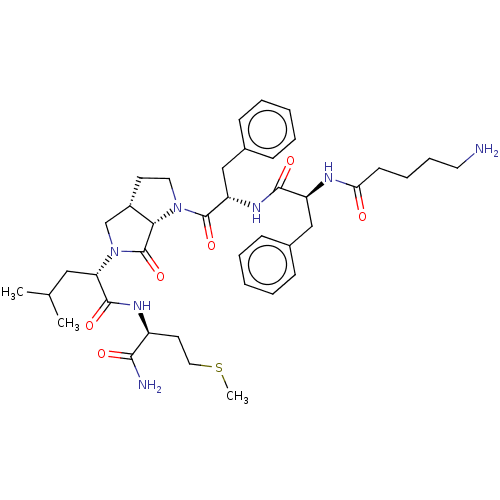 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001604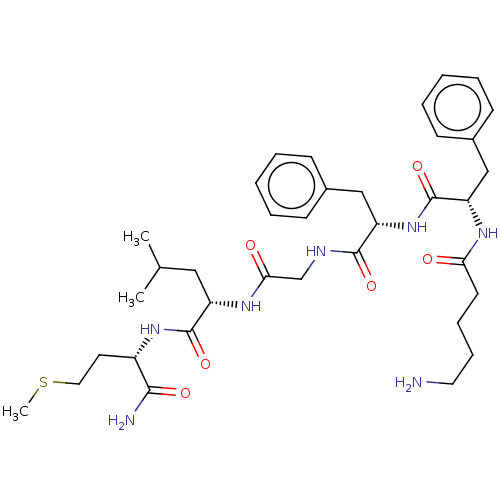 (2-(2-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001603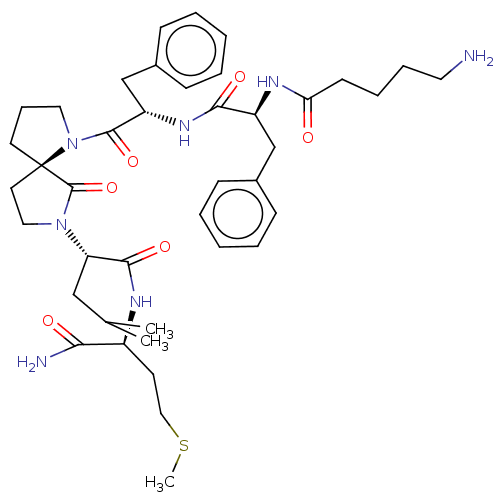 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001598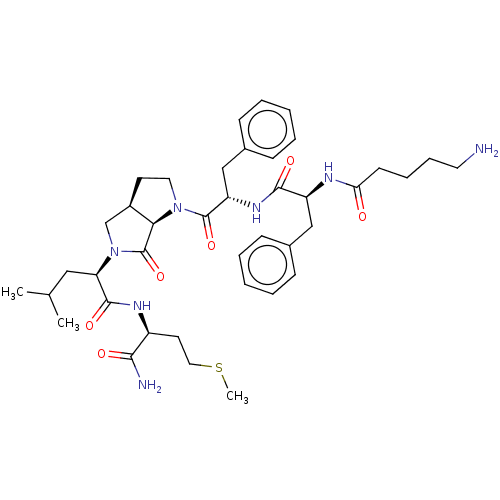 (2-(1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propi...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50001595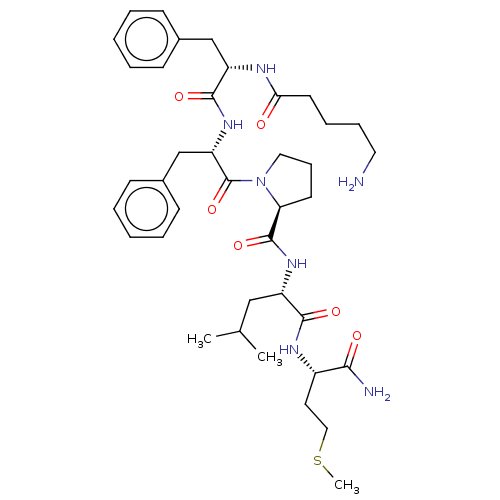 (1-{2-[2-(5-Amino-pentanoylamino)-3-phenyl-propiony...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Glaxo Group Research Curated by ChEMBL | Assay Description In vitro agonistic activity against tachykinin receptor 2 of rat colon muscularis mucosae. | J Med Chem 35: 4195-204 (1992) BindingDB Entry DOI: 10.7270/Q2NZ8883 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052517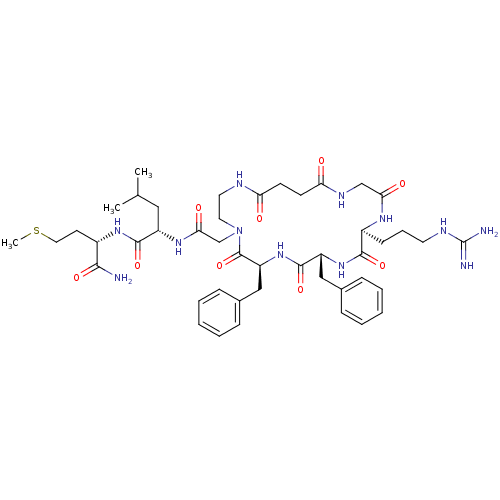 ((S)-2-{2-[(6S,9R,12S)-6,9-Dibenzyl-12-(3-guanidino...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052518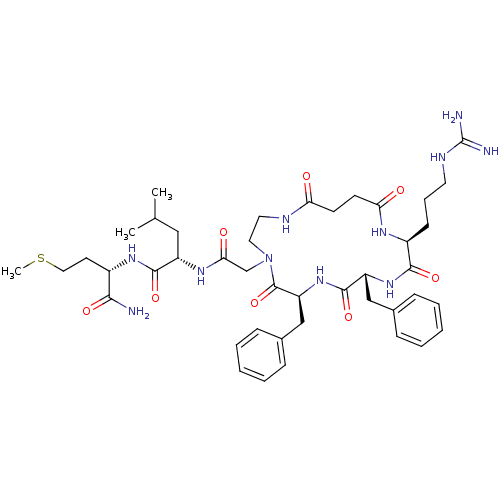 ((S)-2-{2-[(6S,9R,12S)-6,9-Dibenzyl-12-(3-guanidino...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052515 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052520 ((S)-2-{2-[(3S,6R,9S)-3,6-Dibenzyl-9-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052522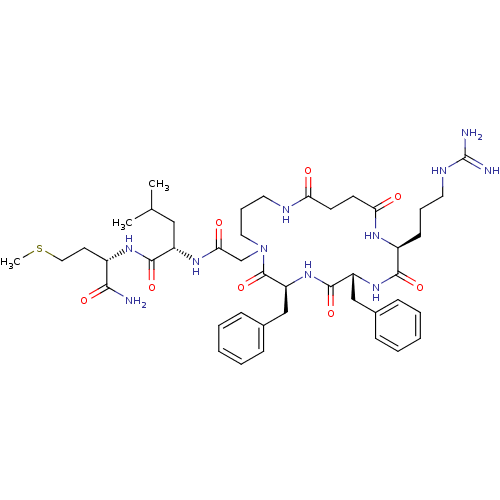 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052516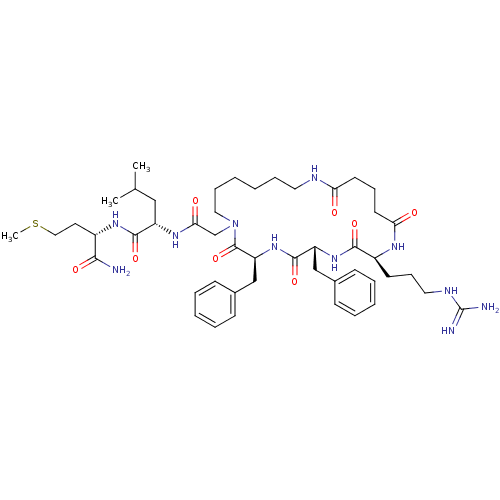 ((S)-2-{2-[(3S,6R,9S)-3,6-Dibenzyl-9-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052525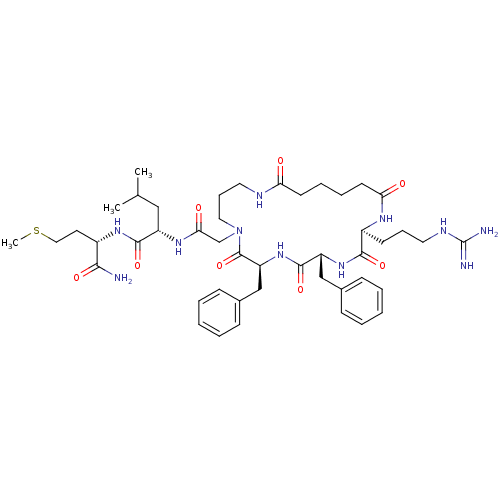 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052519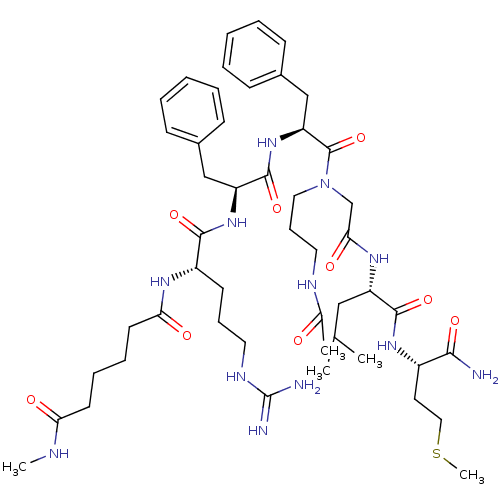 (CHEMBL265115 | Hexanedioic acid (1-{1-[1-((3-acety...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens(RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052521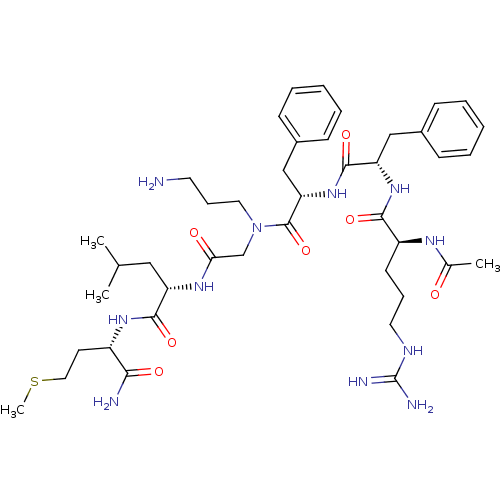 ((S)-2-{2-[{(S)-2-[(S)-2-((S)-2-Acetylamino-5-guani...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052523 ((R)-1-{(S)-2-[(S)-2-((S)-2-Acetylamino-5-guanidino...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052524 ((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||