

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215294 (2-(N-(6-chloro-7-methyl-2,3-dioxo-1,2,3,4-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215283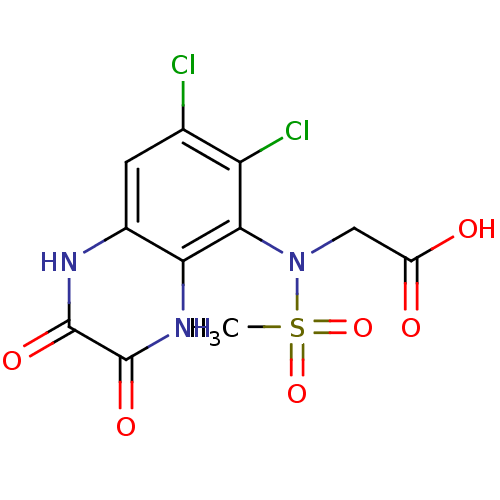 (2-(N-(6,7-dichloro-2,3-dioxo-1,2,3,4-tetrahydroqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215284 (2-(N-(7-chloro-6-methyl-2,3-dioxo-1,2,3,4-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215282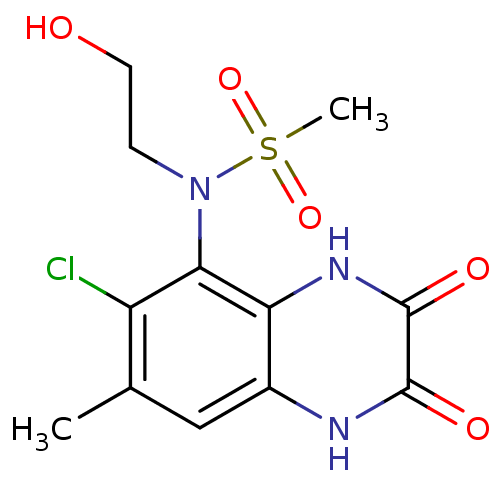 (CHEMBL429296 | N-(6-chloro-7-methyl-2,3-dioxo-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50073922 (CHEMBL86313 | {(S)-1-[(7-Bromo-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091637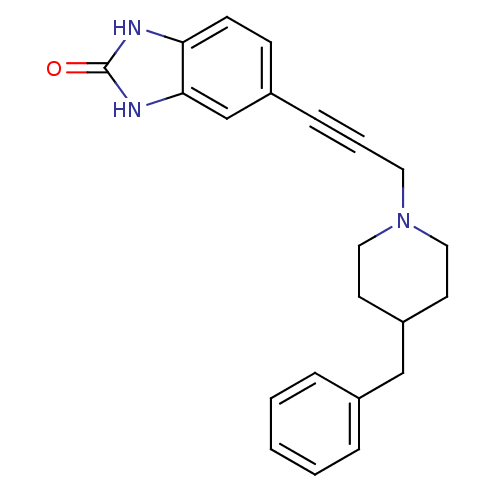 (5-(3-(4-benzylpiperidin-1-yl)prop-1-ynyl)-1H-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50084064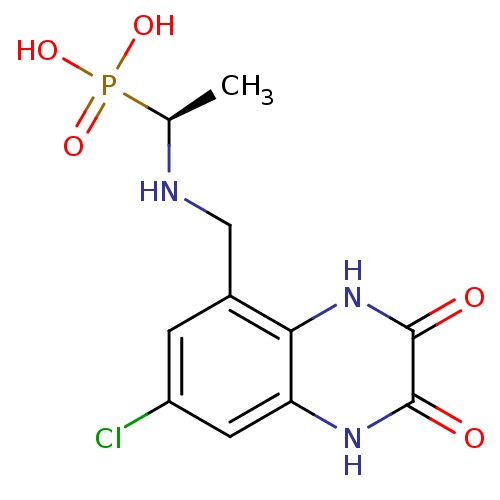 (CHEMBL167952 | {(S)-1-[(7-Chloro-2,3-dioxo-1,2,3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091635 (5-{3-[4-(3-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215286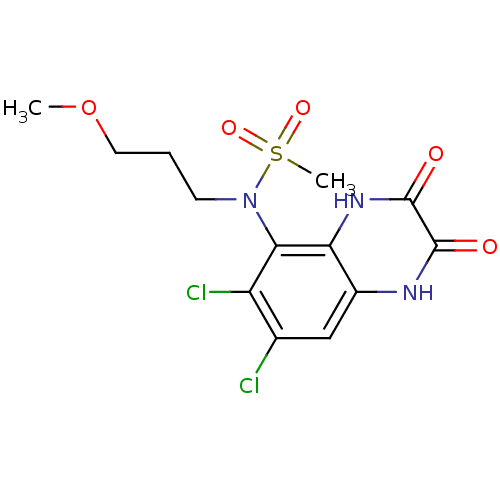 (CHEMBL399275 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50073930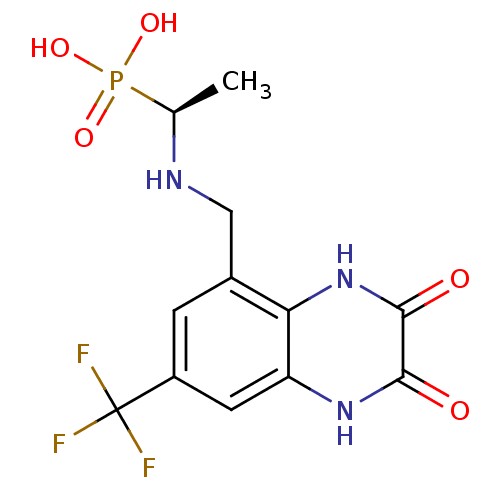 (CHEMBL82963 | {(S)-1-[(2,3-Dioxo-7-trifluoromethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215286 (CHEMBL399275 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50084062 (CHEMBL354295 | {(S)-1-[(7-Iodo-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091636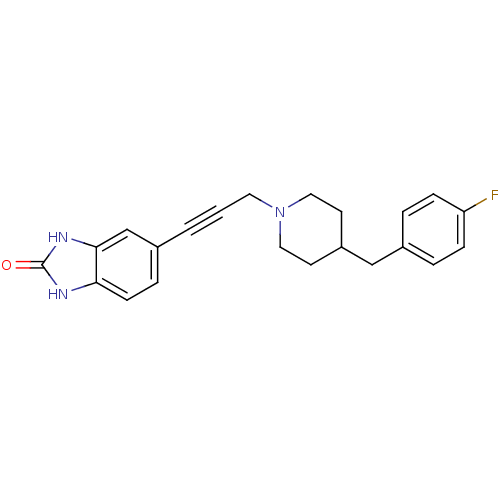 (5-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50084063 (CHEMBL162675 | {(S)-1-[(7-Fluoro-2,3-dioxo-1,2,3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50000663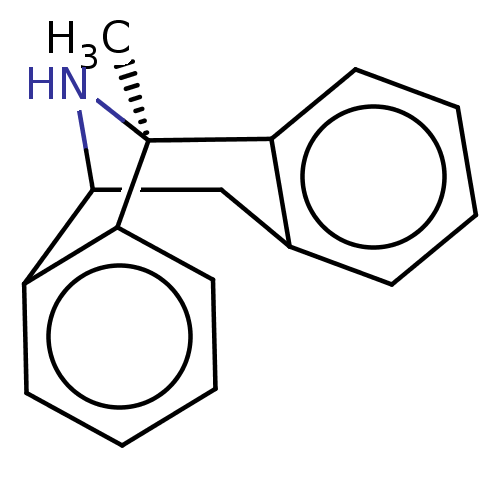 ((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.9 | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Antagonist activity at NR1/2B receptor (unknown origin) expressed in xenopus laevis at pH 6.9 by two electrode voltage clamp method | J Med Chem 51: 3765-76 (2008) Article DOI: 10.1021/jm7011694 BindingDB Entry DOI: 10.7270/Q21Z447W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215281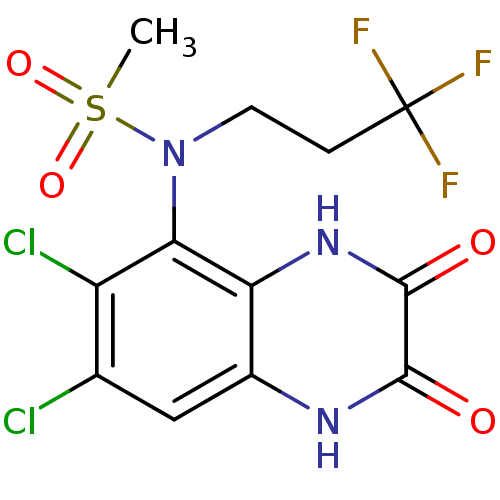 (CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215287 (CHEMBL247035 | N-(3-methoxybenzyl)-N-(6,7-dichloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215296 (CHEMBL401849 | N-(7-chloro-6-methyl-2,3-dioxo-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091647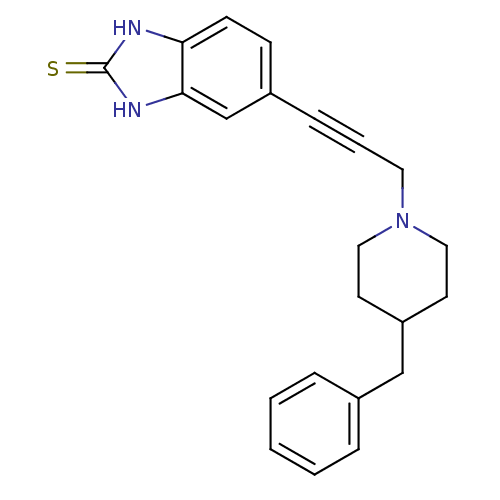 (5-[3-(4-Benzyl-piperidin-1-yl)-prop-1-ynyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091632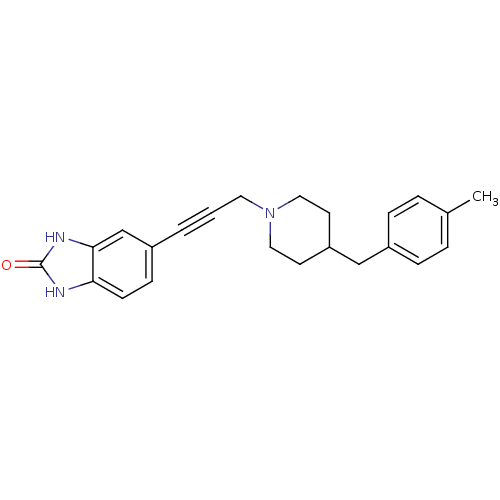 (5-{3-[4-(4-Methyl-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215285 (CHEMBL400508 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091643 (5-{3-[4-(4-Chloro-benzyl)-piperidin-1-yl]-prop-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091626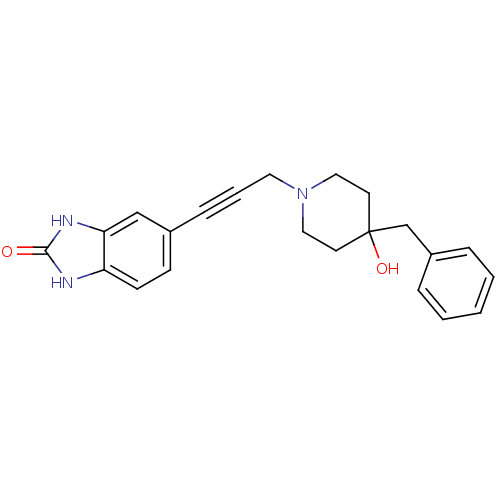 (5-[3-(4-Benzyl-4-hydroxy-piperidin-1-yl)-prop-1-yn...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215276 (CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215281 (CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215281 (CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091628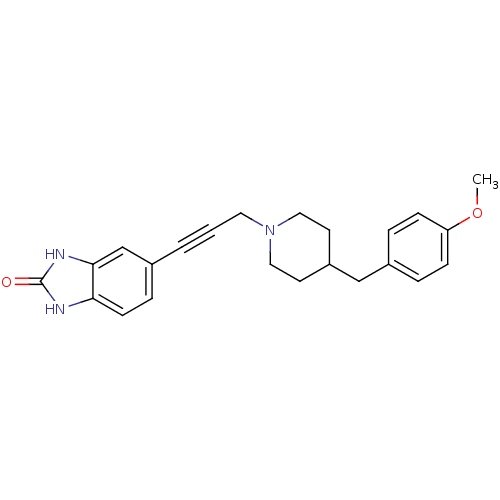 (5-{3-[4-(4-Methoxy-benzyl)-piperidin-1-yl]-prop-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50073950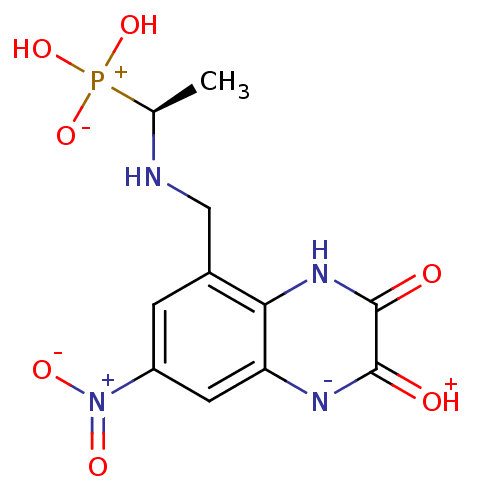 (CHEMBL82626 | {(S)-1-[(7-Nitro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215279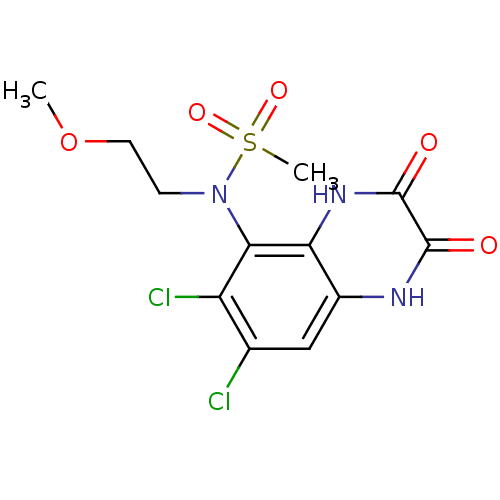 (CHEMBL248439 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215298 (CHEMBL399075 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215297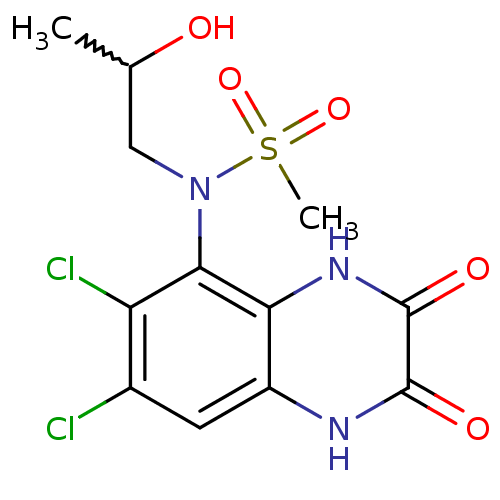 (CHEMBL247034 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215292 (CHEMBL401100 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215280 (CHEMBL248436 | N-(3-fluorobenzyl)-N-(6,7-dichloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215278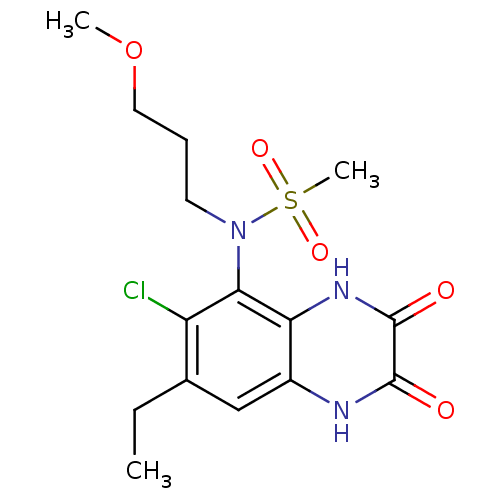 (CHEMBL248446 | N-(6-chloro-7-ethyl-2,3-dioxo-1,2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091645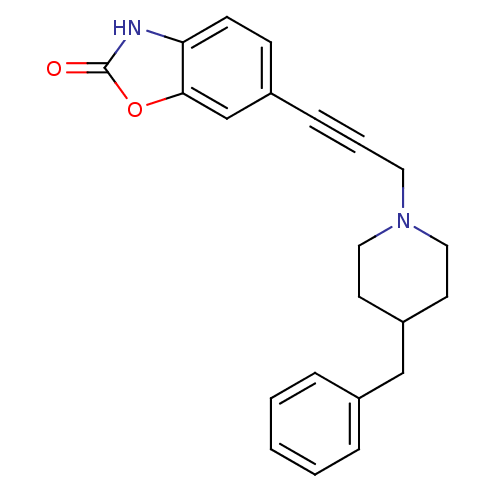 (6-[3-(4-Benzyl-piperidin-1-yl)-prop-1-ynyl]-3H-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215277 (CHEMBL401253 | N-(7-chloro-6-ethyl-2,3-dioxo-1,2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215295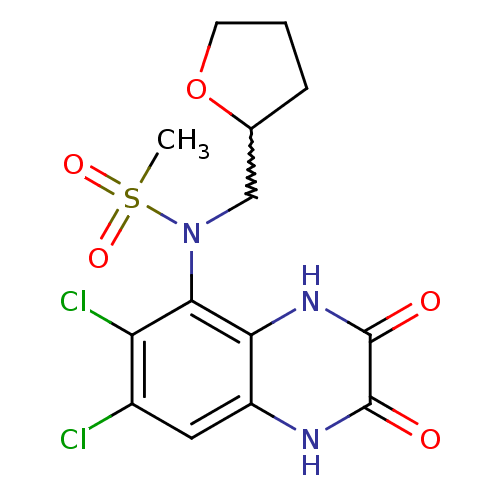 (CHEMBL248438 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215291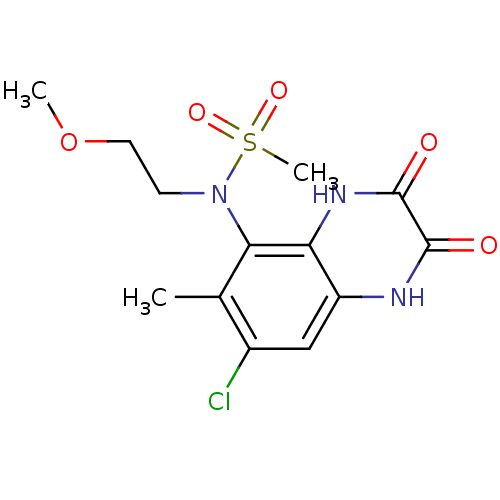 (CHEMBL248445 | N-(7-chloro-6-methyl-2,3-dioxo-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50084065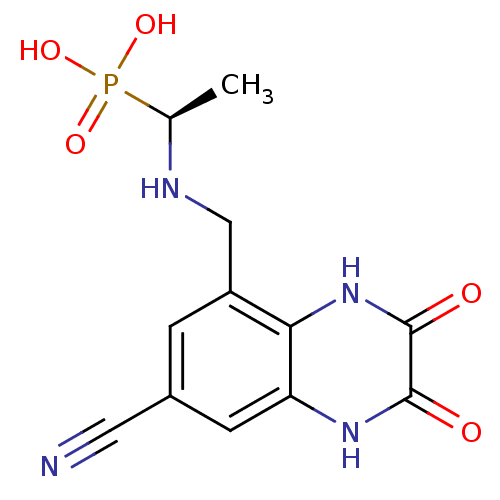 (CHEMBL164231 | {(S)-1-[(7-Cyano-2,3-dioxo-1,2,3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Zurich Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for the glycine binding site on N-methyl-D-aspartate glutamate receptor by using [3H]-MDL-105,519 bin... | Bioorg Med Chem Lett 10: 75-8 (2000) BindingDB Entry DOI: 10.7270/Q2M907WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215288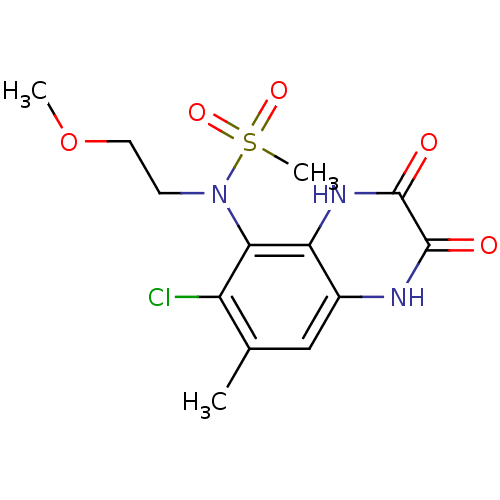 (CHEMBL248444 | N-(6-chloro-7-methyl-2,3-dioxo-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091627 (5-[4-(4-Benzyl-piperidin-1-yl)-but-1-ynyl]-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215276 (CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215276 (CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091634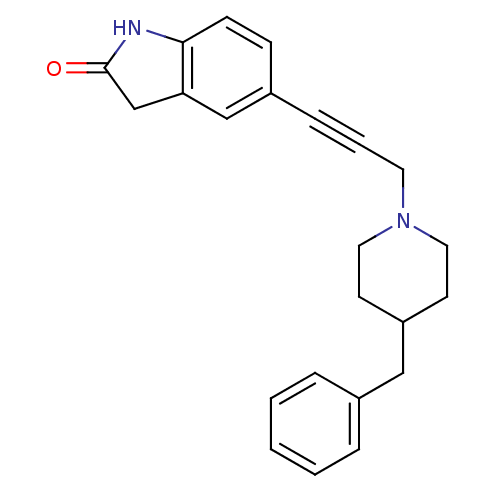 (5-[3-(4-Benzyl-piperidin-1-yl)-prop-1-ynyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091642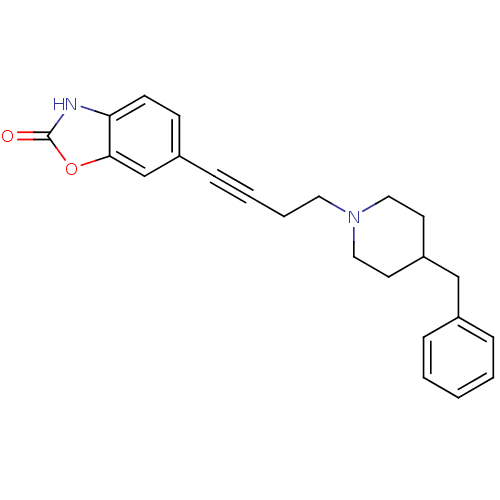 (6-[4-(4-Benzyl-piperidin-1-yl)-but-1-ynyl]-3H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215290 (CHEMBL399274 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091633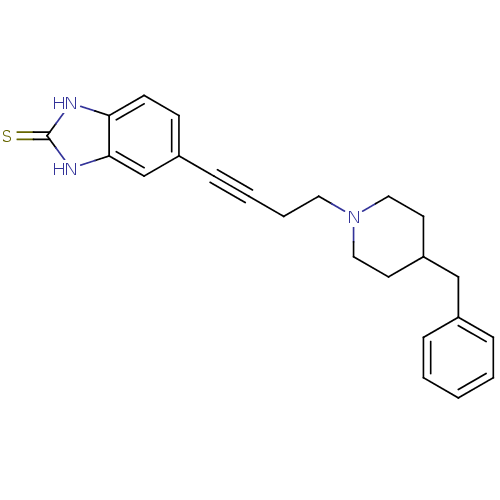 (5-[4-(4-Benzyl-piperidin-1-yl)-but-1-ynyl]-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50215293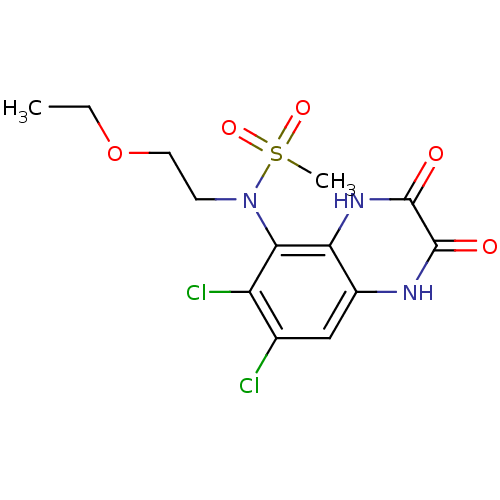 (CHEMBL248442 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human NMDA NR1 receptor | Bioorg Med Chem Lett 17: 4599-603 (2007) Article DOI: 10.1016/j.bmcl.2007.05.083 BindingDB Entry DOI: 10.7270/Q2QR4WTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50091640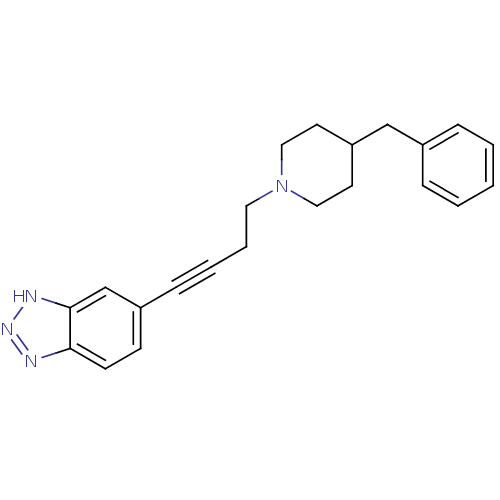 (5-[4-(4-Benzyl-piperidin-1-yl)-but-1-ynyl]-1H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc. Curated by ChEMBL | Assay Description Concentration required for 50% Inhibition of responses at cloned NR1A/2AB NMDA expressed in Xenopus oocytes | J Med Chem 43: 3408-19 (2000) BindingDB Entry DOI: 10.7270/Q2VH5N26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (Homo sapiens (Human)) | BDBM50062613 (17-methyl-(1S,9S,10S)-17-azatetracyclo[7.5.3.01,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | 6.9 | n/a |
Medical University of Lublin Curated by ChEMBL | Assay Description Antagonist activity at NR1/2B receptor (unknown origin) expressed in xenopus laevis at pH 6.9 by two electrode voltage clamp method | J Med Chem 51: 3765-76 (2008) Article DOI: 10.1021/jm7011694 BindingDB Entry DOI: 10.7270/Q21Z447W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |