

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50091652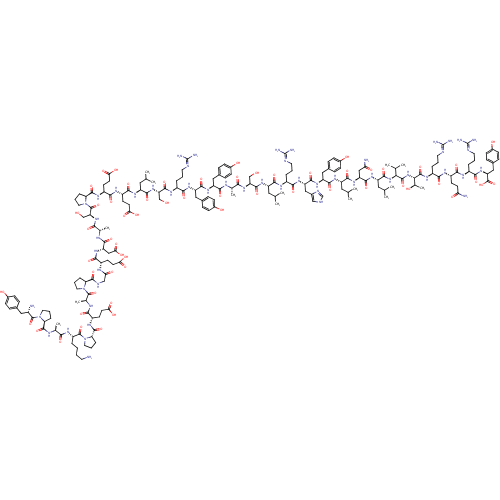 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Medical Center Curated by ChEMBL | Assay Description Affinity against Neuropeptide Y receptor Y1 in SK-N-MC cell line | J Med Chem 43: 3420-7 (2000) BindingDB Entry DOI: 10.7270/Q2QR4WCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 20 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 30 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553107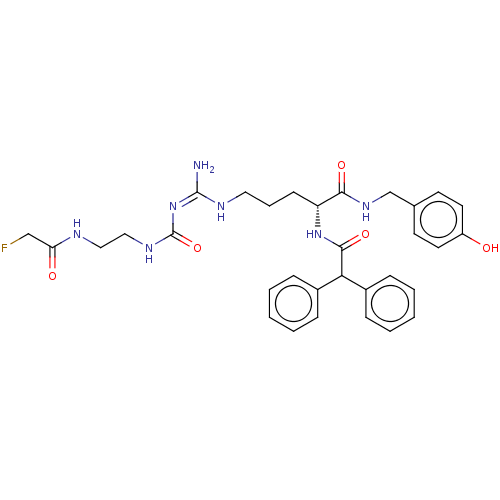 (CHEMBL4746035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553111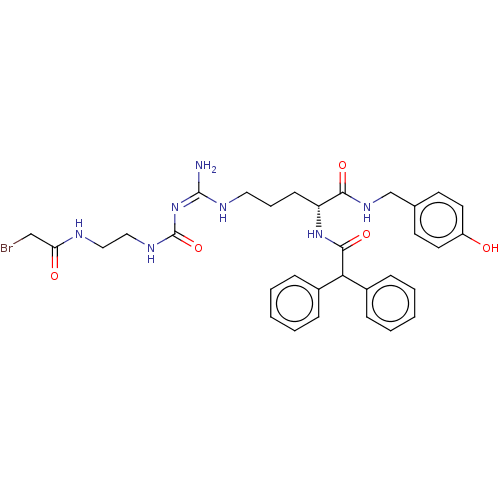 (CHEMBL4786911) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 15 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553117 (CHEMBL4755692) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0661 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490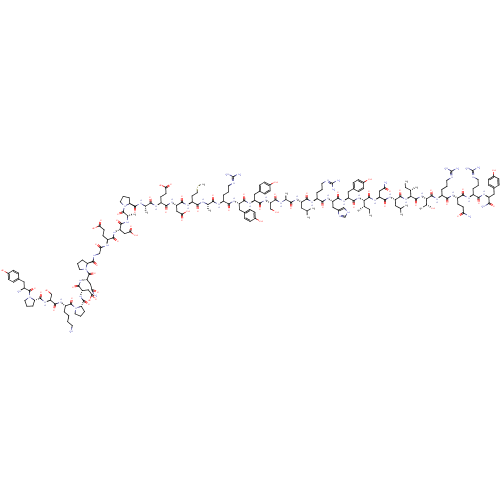 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Binding affinity to human neuropeptide Y1 receptor by radioligand displacement assay | Bioorg Med Chem 21: 2764-71 (2013) Article DOI: 10.1016/j.bmc.2013.03.016 BindingDB Entry DOI: 10.7270/Q2N87C5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553110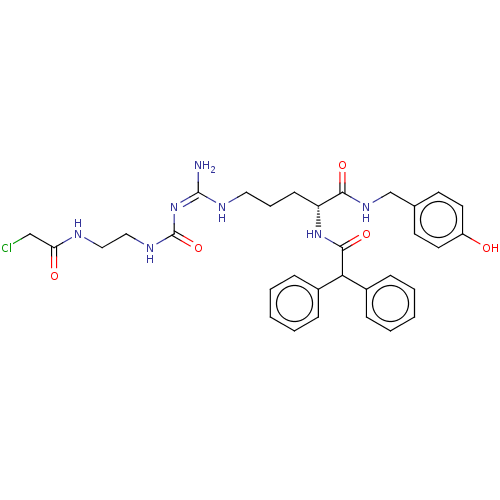 (CHEMBL4794692) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553113 (CHEMBL4798118) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0912 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500154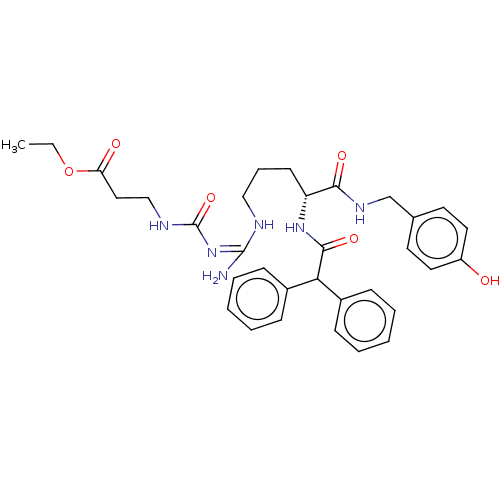 (CHEMBL3746870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 20 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 10 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 30 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500154 (CHEMBL3746870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 15 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553108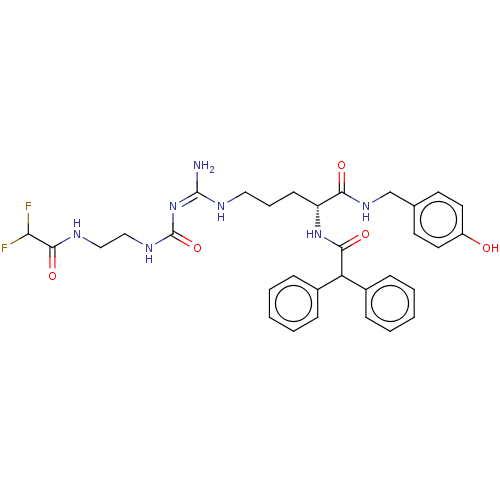 (CHEMBL4743858) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 5 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553109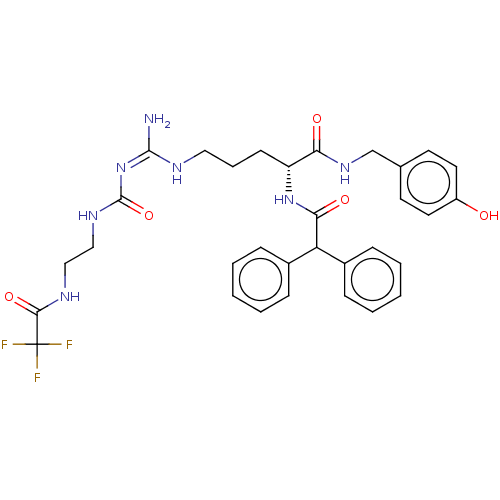 (CHEMBL4751322) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 10 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 20 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301193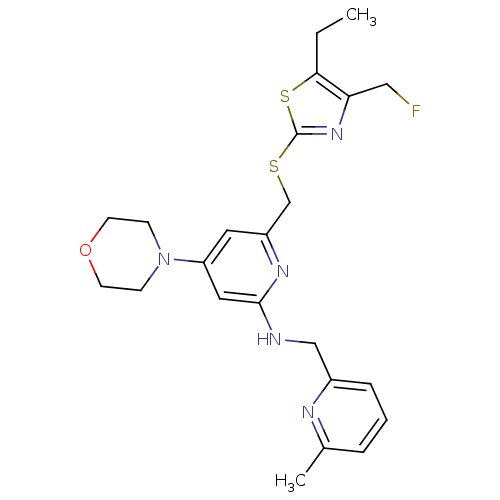 (6-((5-ethyl-4-(fluoromethyl)thiazol-2-ylthio)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [125I]peptide YY from human Y1 receptor after 120 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human neuropeptide Y receptor type 1 by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500154 (CHEMBL3746870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 10 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301204 (6-((4,5-dimethyloxazol-2-ylthio)methyl)-N-((6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500154 (CHEMBL3746870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 5 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 5 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 10 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 5 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553114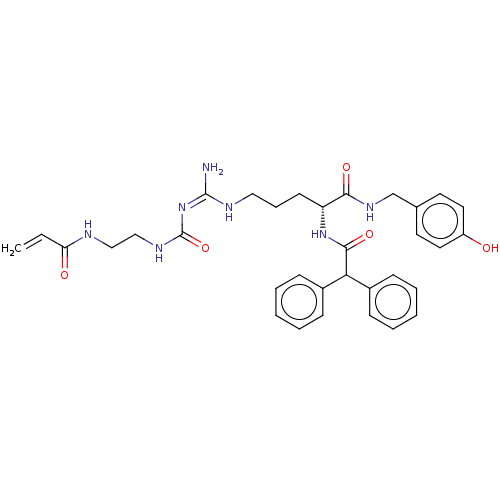 (CHEMBL4753520) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500153 (CHEMBL3746851) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.195 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 15 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301192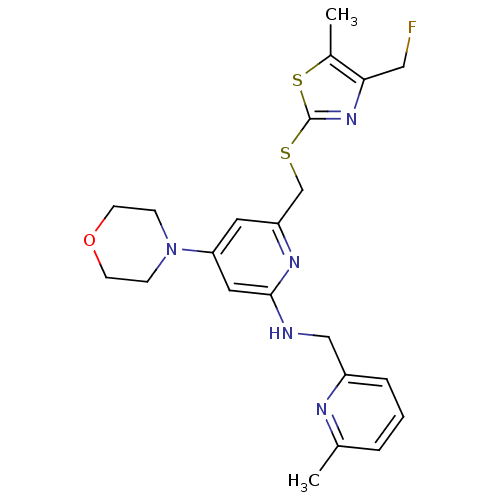 (6-((4-(fluoromethyl)-5-methylthiazol-2-ylthio)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50208988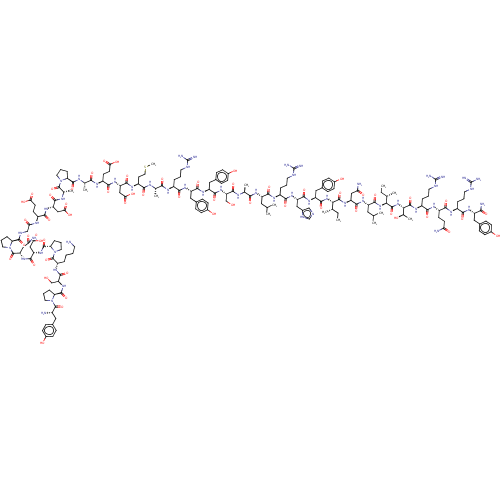 (HUMAN NEUROPEPTIDE Y) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]peptide YY from Y1 receptor in human SK-N-MC cells measured after 120 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500153 (CHEMBL3746851) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.219 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 10 mins by Fura-2 dye bas... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 1 in human SK-N-MC cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 2 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553119 (CHEMBL4745338) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553120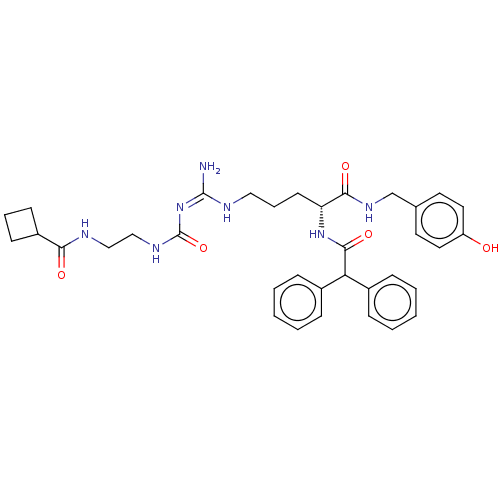 (CHEMBL4762044) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301209 (6-((4,5-dimethylthiazol-2-ylthio)methyl)-N-((6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301203 (6-((4,5-dimethyloxazol-2-ylthio)methyl)-N-((6-fluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553112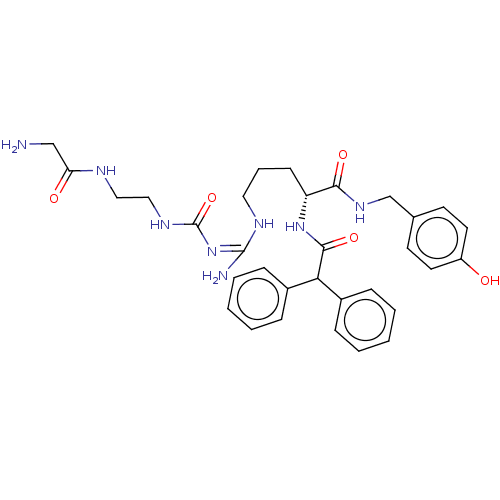 (CHEMBL4752935) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500154 (CHEMBL3746870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 2 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500153 (CHEMBL3746851) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.347 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 5 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301207 (6-((5-ethyl-4-methyloxazol-2-ylthio)methyl)-N-((6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553116 (CHEMBL4789673) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Y1 receptor in human HEL cells assessed as reduction in pNPY-induced calcium mobilization preincubated for 15 mins followed by... | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 2 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 2 mins by Fura-2 dye base... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50301208 (CHEMBL566040 | N-((6-methylpyridin-2-yl)methyl)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human NPYY1 receptor overexpressed in CHO cell membrane after 120 mins by scintillation counting | Bioorg Med Chem Lett 19: 5124-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.030 BindingDB Entry DOI: 10.7270/Q26M36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Antagonist activity at NPY1R in human HEL cells assessed as inhibition of 10 nM pNPY-induced Ca+2 response preincubated for 1 min by Fura-2 dye based... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 586 total ) | Next | Last >> |