 Found 947 hits of ki data for polymerid = 49000892,49000894,49000895
Found 947 hits of ki data for polymerid = 49000892,49000894,49000895 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
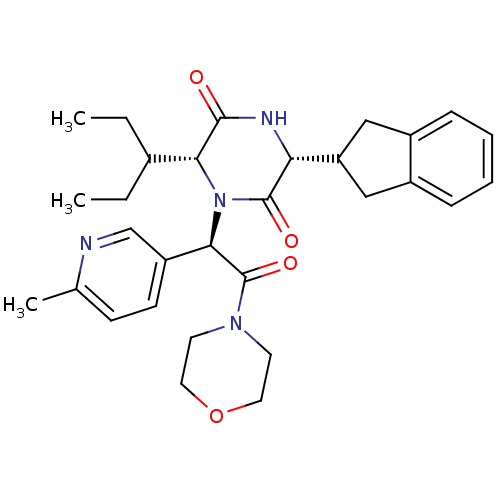
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384800
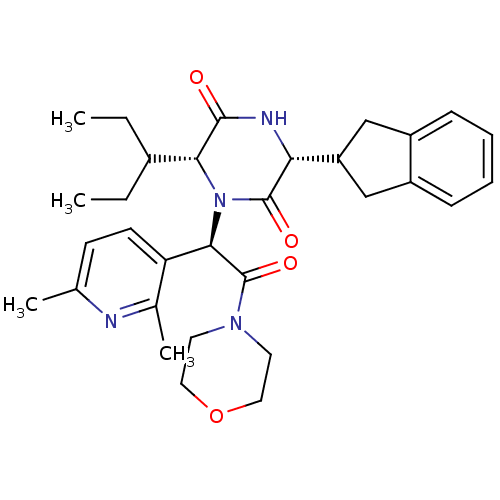
(CHEMBL2037517)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C31H40N4O4/c1-5-21(6-2)27-29(36)33-26(24-17-22-9-7-8-10-23(22)18-24)30(37)35(27)28(25-12-11-19(3)32-20(25)4)31(38)34-13-15-39-16-14-34/h7-12,21,24,26-28H,5-6,13-18H2,1-4H3,(H,33,36)/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384816

(CHEMBL2037516)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H38N4O3/c1-7-19(8-2)25-27(34)31-24(22-15-20-11-9-10-12-21(20)16-22)28(35)33(25)26(29(36)32(5)6)23-14-13-17(3)30-18(23)4/h9-14,19,22,24-26H,7-8,15-16H2,1-6H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384823
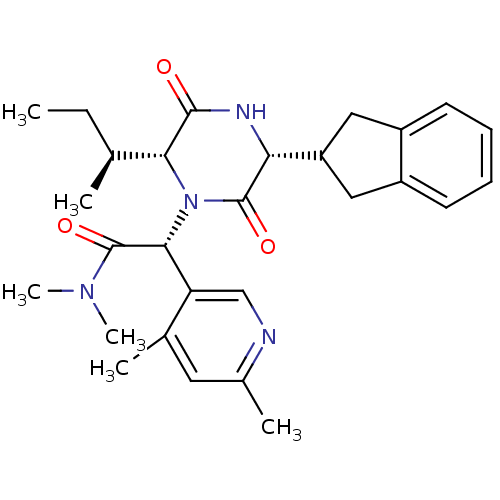
(CHEMBL2037507)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-15-29-18(4)12-17(22)3/h8-12,15-16,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384818
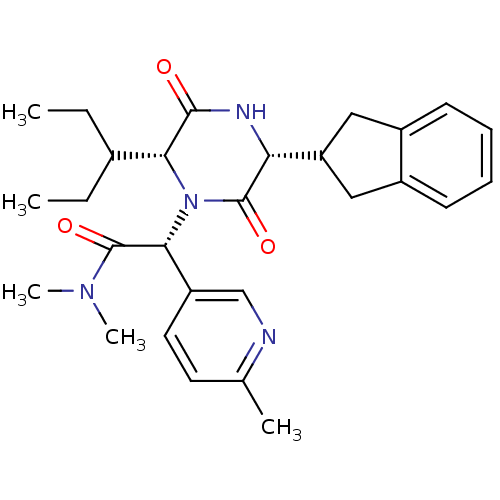
(CHEMBL2037513)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-26(33)30-23(22-14-19-10-8-9-11-20(19)15-22)27(34)32(24)25(28(35)31(4)5)21-13-12-17(3)29-16-21/h8-13,16,18,22-25H,6-7,14-15H2,1-5H3,(H,30,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384837

(CHEMBL2037515)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-27(34)31-23(21-14-19-10-8-9-11-20(19)15-21)28(35)32(24)25(26(33)29-5)22-13-12-16(3)30-17(22)4/h8-13,18,21,23-25H,6-7,14-15H2,1-5H3,(H,29,33)(H,31,34)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384822
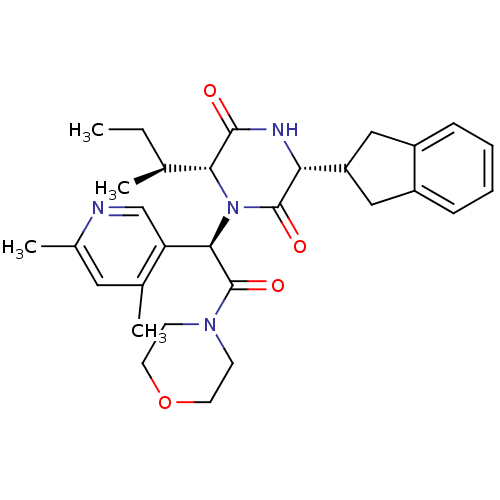
(CHEMBL2037508)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-17-31-20(4)14-19(24)3)30(37)33-10-12-38-13-11-33/h6-9,14,17-18,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384815
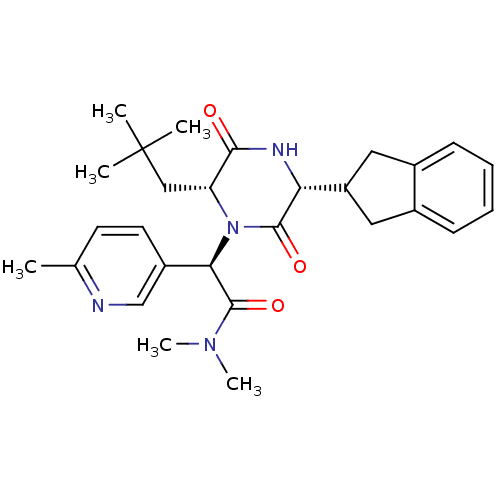
(CHEMBL2037496)Show SMILES CN(C)C(=O)[C@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)c1ccc(C)nc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-17-11-12-20(16-29-17)24(27(35)31(5)6)32-22(15-28(2,3)4)25(33)30-23(26(32)34)21-13-18-9-7-8-10-19(18)14-21/h7-12,16,21-24H,13-15H2,1-6H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384824
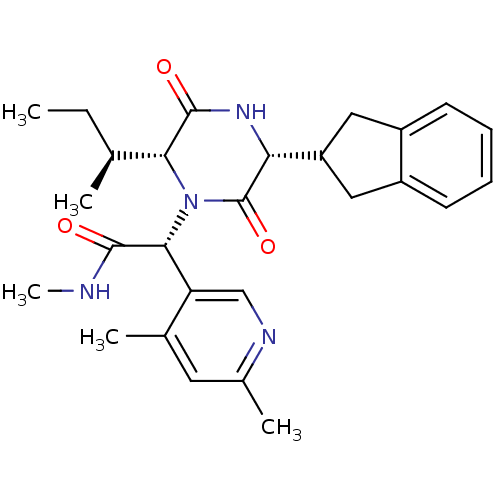
(CHEMBL2037506)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-14-29-17(4)11-16(21)3/h7-11,14-15,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384834
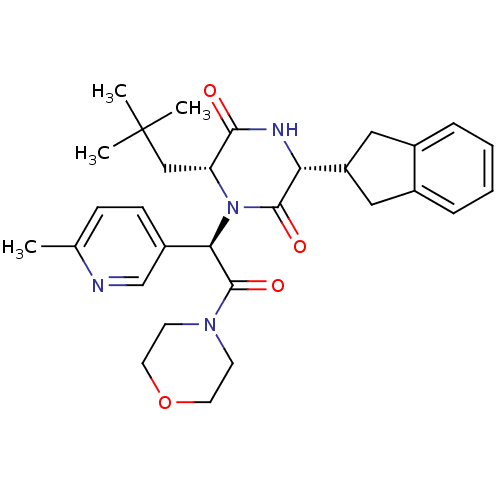
(CHEMBL2037497)Show SMILES Cc1ccc(cn1)[C@@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C30H38N4O4/c1-19-9-10-22(18-31-19)26(29(37)33-11-13-38-14-12-33)34-24(17-30(2,3)4)27(35)32-25(28(34)36)23-15-20-7-5-6-8-21(20)16-23/h5-10,18,23-26H,11-17H2,1-4H3,(H,32,35)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50190528
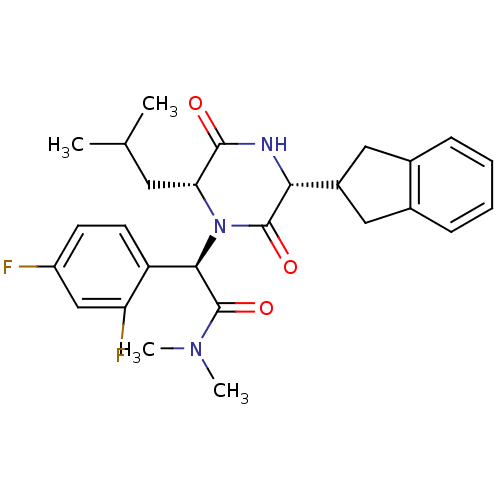
((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H31F2N3O3/c1-15(2)11-22-25(33)30-23(18-12-16-7-5-6-8-17(16)13-18)26(34)32(22)24(27(35)31(3)4)20-10-9-19(28)14-21(20)29/h5-10,14-15,18,22-24H,11-13H2,1-4H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384838
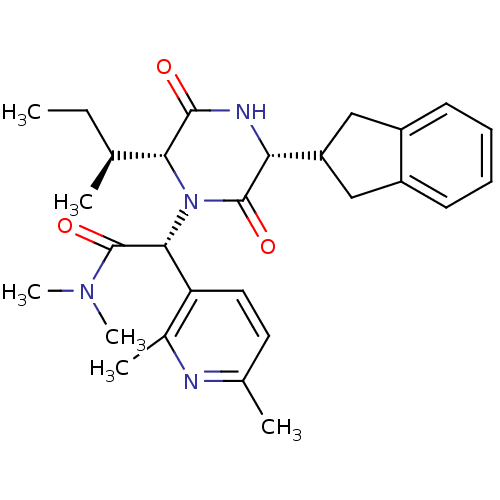
(CHEMBL2037510)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-14-19-10-8-9-11-20(19)15-21)27(34)32(24)25(28(35)31(5)6)22-13-12-17(3)29-18(22)4/h8-13,16,21,23-25H,7,14-15H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370109

(CHEMBL1790723)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6](-[#8])-[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(-[#6]-2=[#6]-3-[#6]=[#6]-[#6](=[#6]-[#6]-3-[#8]-[#6]-3=[#6]\[#6](-[#6]=[#6]-[#6]-2-3)=[#7+](\[#6])-[#6])-[#7](-[#6])-[#6])c(c1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#7])-c1ccc(-[#8])cc1 |c:62,64,66,74,t:71| Show InChI InChI=1S/C65H84N14O16S2/c1-7-33(2)54-62(90)72-44(22-23-51(67)82)58(86)73-45(29-52(68)83)59(87)74-46(30-96-97-31-48(81)61(89)76-55(63(91)75-54)34-12-17-38(80)18-13-34)64(92)79-25-9-11-47(79)60(88)71-43(57(85)70-32-66)10-8-24-69-56(84)35-14-19-39(42(26-35)65(93)94)53-40-20-15-36(77(3)4)27-49(40)95-50-28-37(78(5)6)16-21-41(50)53/h12-21,26-28,33,40,43-48,50,54-55,81H,7-11,22-25,29-32,66H2,1-6H3,(H13-,67,68,69,70,71,72,73,74,75,76,80,82,83,84,85,86,87,88,89,90,91,93,94)/p+1/t33-,40?,43+,44-,45-,46-,47-,48?,50?,54-,55+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384819
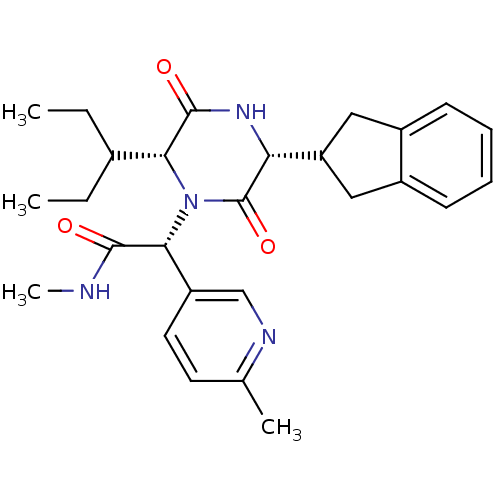
(CHEMBL2037512)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-5-17(6-2)23-26(33)30-22(21-13-18-9-7-8-10-19(18)14-21)27(34)31(23)24(25(32)28-4)20-12-11-16(3)29-15-20/h7-12,15,17,21-24H,5-6,13-14H2,1-4H3,(H,28,32)(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384820
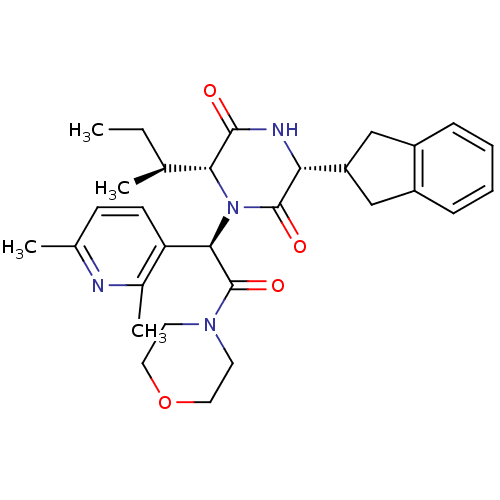
(EPELSIBAN | GSK557296B)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384803
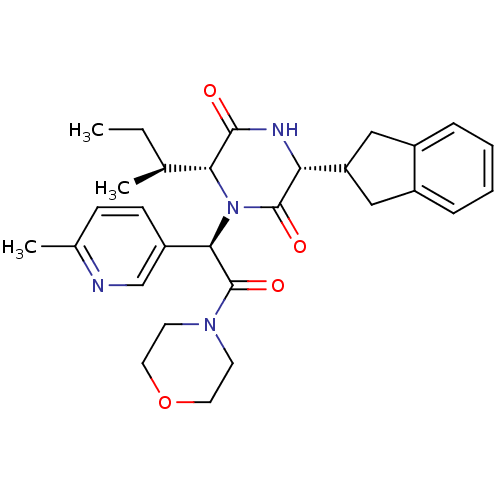
(CHEMBL2037501)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370113

(CHEMBL1790719)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C60H71N11O18S2/c1-4-28(2)47-55(83)69-48(29(3)72)56(84)66-40(24-46(62)77)52(80)67-41(25-90-91-26-43(76)54(82)70-49(57(85)68-47)30-9-12-32(73)13-10-30)58(86)71-20-6-8-42(71)53(81)65-39(51(79)64-27-61)7-5-19-63-50(78)31-11-16-35-38(21-31)60(89-59(35)87)36-17-14-33(74)22-44(36)88-45-23-34(75)15-18-37(45)60/h9-18,21-23,28-29,39-43,47-49,72-76H,4-8,19-20,24-27,61H2,1-3H3,(H2,62,77)(H,63,78)(H,64,79)(H,65,81)(H,66,84)(H,67,80)(H,68,85)(H,69,83)(H,70,82)/t28-,29?,39+,40-,41-,42-,43?,47-,48-,49+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370117

(CHEMBL1790720)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H72N12O18S2/c1-3-29(2)49-57(86)68-40(18-19-47(63)78)53(82)69-41(25-48(64)79)54(83)70-42(26-92-93-27-44(77)56(85)72-50(58(87)71-49)30-8-11-32(74)12-9-30)59(88)73-21-5-7-43(73)55(84)67-39(52(81)66-28-62)6-4-20-65-51(80)31-10-15-35-38(22-31)61(91-60(35)89)36-16-13-33(75)23-45(36)90-46-24-34(76)14-17-37(46)61/h8-17,22-24,29,39-44,49-50,74-77H,3-7,18-21,25-28,62H2,1-2H3,(H2,63,78)(H2,64,79)(H,65,80)(H,66,81)(H,67,84)(H,68,86)(H,69,82)(H,70,83)(H,71,87)(H,72,85)/t29-,39+,40-,41-,42-,43-,44?,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384805
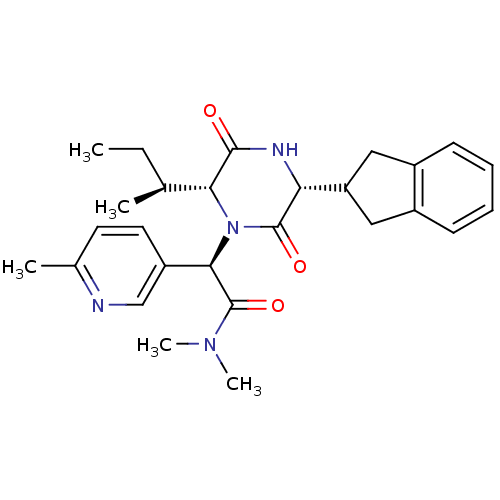
(CHEMBL2037499)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370106

(CHEMBL1790712)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@](C)(NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@H](C(N)=O)c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C52H75N11O13S2/c1-5-28(2)40-47(73)59-41(29(3)64)48(74)57-35(25-38(54)67)45(71)58-36(27-77-78-52(21-7-6-8-22-52)26-39(68)62-51(4,50(76)61-40)31-15-19-33(66)20-16-31)49(75)63-24-10-12-37(63)46(72)56-34(11-9-23-53)44(70)60-42(43(55)69)30-13-17-32(65)18-14-30/h13-20,28-29,34-37,40-42,64-66H,5-12,21-27,53H2,1-4H3,(H2,54,67)(H2,55,69)(H,56,72)(H,57,74)(H,58,71)(H,59,73)(H,60,70)(H,61,76)(H,62,68)/t28-,29-,34+,35-,36-,37-,40-,41-,42+,51-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077141
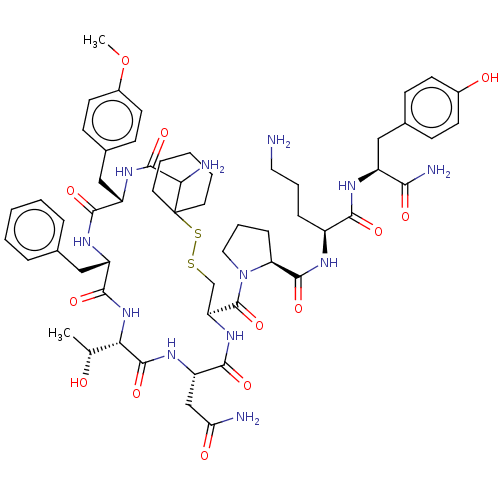
(CHEMBL3416758)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)C(N)C2(CCCCC2)SSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)[C@@H](C)O |r| Show InChI InChI=1S/C57H78N12O13S2/c1-32(70)46-54(79)65-42(30-45(59)72)51(76)67-43(56(81)69-26-10-14-44(69)53(78)62-38(13-9-25-58)49(74)63-39(48(61)73)27-34-15-19-36(71)20-16-34)31-83-84-57(23-7-4-8-24-57)47(60)55(80)66-40(29-35-17-21-37(82-2)22-18-35)50(75)64-41(52(77)68-46)28-33-11-5-3-6-12-33/h3,5-6,11-12,15-22,32,38-44,46-47,70-71H,4,7-10,13-14,23-31,58,60H2,1-2H3,(H2,59,72)(H2,61,73)(H,62,78)(H,63,74)(H,64,75)(H,65,79)(H,66,80)(H,67,76)(H,68,77)/t32-,38+,39+,40+,41+,42+,43+,44+,46+,47?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Competitive binding to human oxytocin receptor by radioligand binding assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370110

(CHEMBL1790721)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H72N12O17S2/c1-3-30(2)50-57(85)68-41(18-19-47(63)77)54(82)69-42(27-48(64)78)55(83)70-43(28-92-91-23-20-49(79)71-51(58(86)72-50)31-8-11-33(74)12-9-31)59(87)73-22-5-7-44(73)56(84)67-40(53(81)66-29-62)6-4-21-65-52(80)32-10-15-36-39(24-32)61(90-60(36)88)37-16-13-34(75)25-45(37)89-46-26-35(76)14-17-38(46)61/h8-17,24-26,30,40-44,50-51,74-76H,3-7,18-23,27-29,62H2,1-2H3,(H2,63,77)(H2,64,78)(H,65,80)(H,66,81)(H,67,84)(H,68,85)(H,69,82)(H,70,83)(H,71,79)(H,72,86)/t30-,40+,41-,42-,43-,44-,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384812
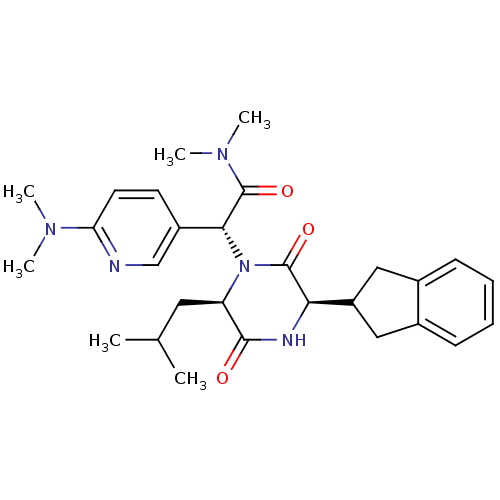
(CHEMBL2037489)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H37N5O3/c1-17(2)13-22-26(34)30-24(21-14-18-9-7-8-10-19(18)15-21)27(35)33(22)25(28(36)32(5)6)20-11-12-23(29-16-20)31(3)4/h7-12,16-17,21-22,24-25H,13-15H2,1-6H3,(H,30,34)/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384836
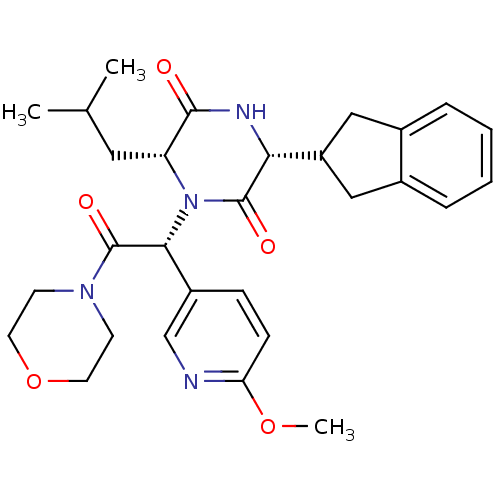
(CHEMBL2037487)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C29H36N4O5/c1-18(2)14-23-27(34)31-25(22-15-19-6-4-5-7-20(19)16-22)28(35)33(23)26(21-8-9-24(37-3)30-17-21)29(36)32-10-12-38-13-11-32/h4-9,17-18,22-23,25-26H,10-16H2,1-3H3,(H,31,34)/t23-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370114
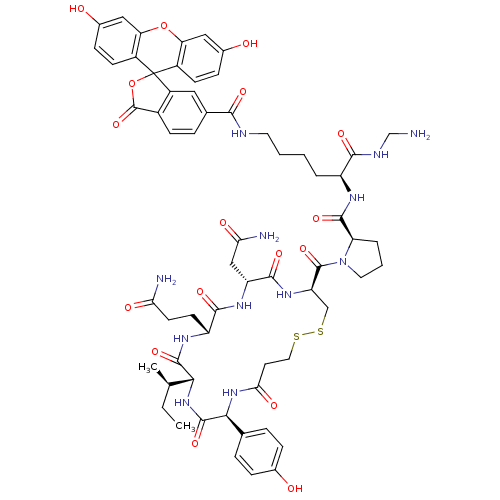
(CHEMBL1790718)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C62H74N12O17S2/c1-3-31(2)51-58(86)69-42(19-20-48(64)78)55(83)70-43(28-49(65)79)56(84)71-44(29-93-92-24-21-50(80)72-52(59(87)73-51)32-9-12-34(75)13-10-32)60(88)74-23-6-8-45(74)57(85)68-41(54(82)67-30-63)7-4-5-22-66-53(81)33-11-16-37-40(25-33)62(91-61(37)89)38-17-14-35(76)26-46(38)90-47-27-36(77)15-18-39(47)62/h9-18,25-27,31,41-45,51-52,75-77H,3-8,19-24,28-30,63H2,1-2H3,(H2,64,78)(H2,65,79)(H,66,81)(H,67,82)(H,68,85)(H,69,86)(H,70,83)(H,71,84)(H,72,80)(H,73,87)/t31-,41+,42-,43-,44-,45-,51-,52+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50332720
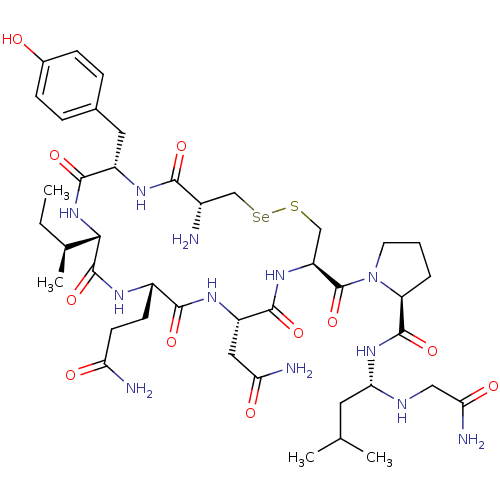
((S)-1-((4R,7S,10S,13S,16S,19R)-4-amino-16-(2-amino...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)C[Se]SC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)NCC(N)=O |r| Show InChI InChI=1S/C42H66N12O11SSe/c1-5-22(4)35-41(64)48-26(12-13-31(44)56)37(60)50-28(17-32(45)57)38(61)51-29(42(65)54-14-6-7-30(54)40(63)52-34(15-21(2)3)47-18-33(46)58)19-66-67-20-25(43)36(59)49-27(39(62)53-35)16-23-8-10-24(55)11-9-23/h8-11,21-22,25-30,34-35,47,55H,5-7,12-20,43H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,48,64)(H,49,59)(H,50,60)(H,51,61)(H,52,63)(H,53,62)/t22-,25-,26-,27-,28-,29-,30-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from oxytocin receptor expressed in COS1 cells |
J Med Chem 53: 8585-96 (2010)
Article DOI: 10.1021/jm100989w
BindingDB Entry DOI: 10.7270/Q2CC10X9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103475

(Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe(I,N3)-NH2 | Pm...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C57H84IN15O13S2/c1-5-31(2)47(69-52(81)40(26-33-13-16-35(86-4)17-14-33)64-46(77)29-57(20-7-6-8-21-57)88-24-19-44(60)75)54(83)70-48(32(3)74)55(84)67-41(28-45(61)76)51(80)68-42(30-87)56(85)73-23-10-12-43(73)53(82)65-38(11-9-22-59)50(79)66-39(49(62)78)27-34-15-18-37(71-72-63)36(58)25-34/h13-18,25,31-32,38-43,47-48,74,87H,5-12,19-24,26-30,59H2,1-4H3,(H2,60,75)(H2,61,76)(H2,62,78)(H,64,77)(H,65,82)(H,66,79)(H,67,84)(H,68,80)(H,69,81)(H,70,83)/t31-,32+,38?,39?,40-,41-,42-,43?,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity towards OT receptor in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384811
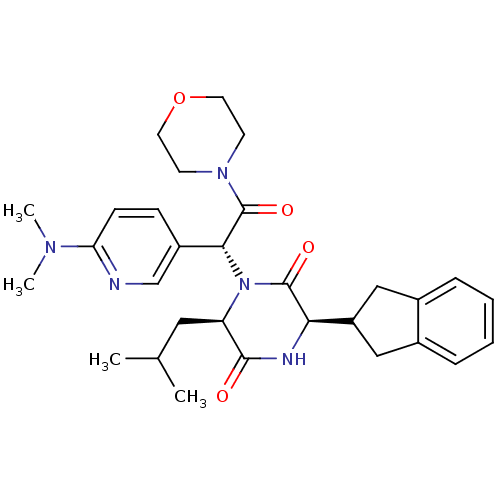
(CHEMBL2037490)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H39N5O4/c1-19(2)15-24-28(36)32-26(23-16-20-7-5-6-8-21(20)17-23)29(37)35(24)27(30(38)34-11-13-39-14-12-34)22-9-10-25(31-18-22)33(3)4/h5-10,18-19,23-24,26-27H,11-17H2,1-4H3,(H,32,36)/t24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370112

(CHEMBL1790729)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H73N11O18S2/c1-4-29(2)48-56(84)70-49(30(3)73)57(85)67-41(25-47(63)78)53(81)68-42(26-91-92-27-44(77)55(83)71-50(58(86)69-48)31-10-13-33(74)14-11-31)59(87)72-21-7-9-43(72)54(82)66-40(52(80)65-28-62)8-5-6-20-64-51(79)32-12-17-36-39(22-32)61(90-60(36)88)37-18-15-34(75)23-45(37)89-46-24-35(76)16-19-38(46)61/h10-19,22-24,29-30,40-44,48-50,73-77H,4-9,20-21,25-28,62H2,1-3H3,(H2,63,78)(H,64,79)(H,65,80)(H,66,82)(H,67,85)(H,68,81)(H,69,86)(H,70,84)(H,71,83)/t29-,30?,40+,41-,42-,43-,44?,48-,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044676
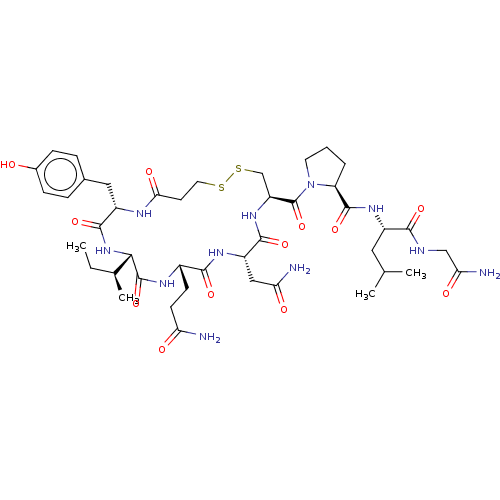
(CHEMBL439044)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC Show InChI InChI=1S/C43H65N11O12S2/c1-5-23(4)36-42(65)49-26(12-13-32(44)56)38(61)50-29(19-33(45)57)39(62)52-30(21-68-67-16-14-35(59)48-28(40(63)53-36)18-24-8-10-25(55)11-9-24)43(66)54-15-6-7-31(54)41(64)51-27(17-22(2)3)37(60)47-20-34(46)58/h8-11,22-23,26-31,36,55H,5-7,12-21H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,47,60)(H,48,59)(H,49,65)(H,50,61)(H,51,64)(H,52,62)(H,53,63)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... |
Bioorg Med Chem 24: 3513-20 (2016)
Article DOI: 10.1016/j.bmc.2016.05.062
BindingDB Entry DOI: 10.7270/Q2WH2RXT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370115
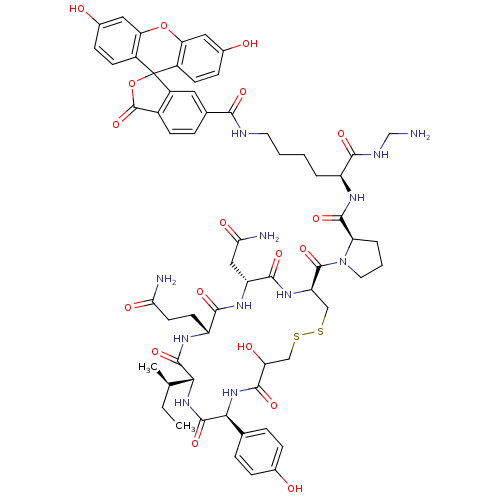
(CHEMBL1790717)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C62H74N12O18S2/c1-3-30(2)50-58(87)69-41(19-20-48(64)79)54(83)70-42(26-49(65)80)55(84)71-43(27-93-94-28-45(78)57(86)73-51(59(88)72-50)31-9-12-33(75)13-10-31)60(89)74-22-6-8-44(74)56(85)68-40(53(82)67-29-63)7-4-5-21-66-52(81)32-11-16-36-39(23-32)62(92-61(36)90)37-17-14-34(76)24-46(37)91-47-25-35(77)15-18-38(47)62/h9-18,23-25,30,40-45,50-51,75-78H,3-8,19-22,26-29,63H2,1-2H3,(H2,64,79)(H2,65,80)(H,66,81)(H,67,82)(H,68,85)(H,69,87)(H,70,83)(H,71,84)(H,72,88)(H,73,86)/t30-,40+,41-,42-,43-,44-,45?,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384821
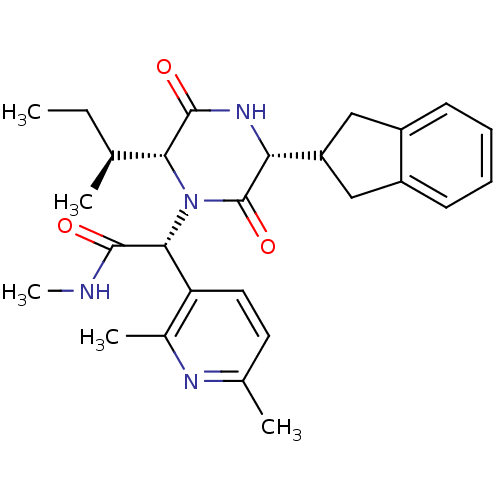
(CHEMBL2037509)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-13-18-9-7-8-10-19(18)14-20)27(34)31(23)24(25(32)28-5)21-12-11-16(3)29-17(21)4/h7-12,15,20,22-24H,6,13-14H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384814
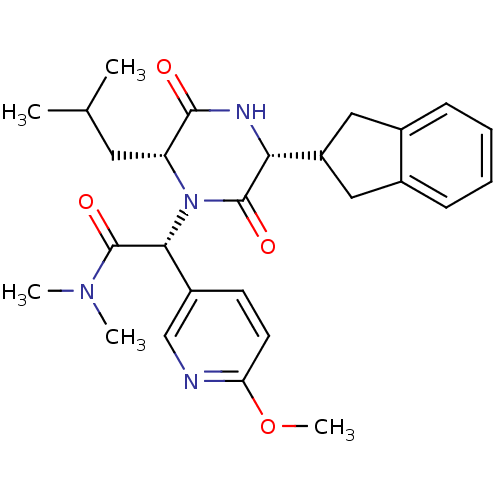
(CHEMBL2037486)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N(C)C |r| Show InChI InChI=1S/C27H34N4O4/c1-16(2)12-21-25(32)29-23(20-13-17-8-6-7-9-18(17)14-20)26(33)31(21)24(27(34)30(3)4)19-10-11-22(35-5)28-15-19/h6-11,15-16,20-21,23-24H,12-14H2,1-5H3,(H,29,32)/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384835
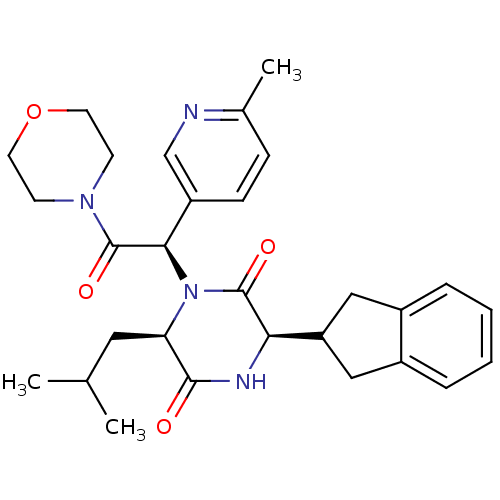
(CHEMBL2037492)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)14-24-27(34)31-25(23-15-20-6-4-5-7-21(20)16-23)28(35)33(24)26(22-9-8-19(3)30-17-22)29(36)32-10-12-37-13-11-32/h4-9,17-18,23-26H,10-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370111

(CHEMBL1790711)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)[C@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C60H71N11O17S2/c1-4-29(2)48-55(82)70-49(30(3)72)56(83)66-41(26-46(62)76)53(80)67-42(27-90-89-22-19-47(77)68-50(57(84)69-48)31-9-12-33(73)13-10-31)58(85)71-21-6-8-43(71)54(81)65-40(52(79)64-28-61)7-5-20-63-51(78)32-11-16-36-39(23-32)60(88-59(36)86)37-17-14-34(74)24-44(37)87-45-25-35(75)15-18-38(45)60/h9-18,23-25,29-30,40-43,48-50,72-75H,4-8,19-22,26-28,61H2,1-3H3,(H2,62,76)(H,63,78)(H,64,79)(H,65,81)(H,66,83)(H,67,80)(H,68,77)(H,69,84)(H,70,82)/t29-,30+,40+,41-,42-,43-,48-,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50305511
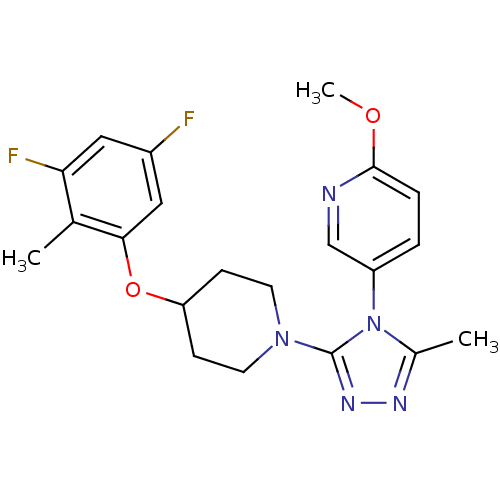
(5-(3-(4-(3,5-difluoro-2-methylphenoxy)piperidin-1-...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1N1CCC(CC1)Oc1cc(F)cc(F)c1C Show InChI InChI=1S/C21H23F2N5O2/c1-13-18(23)10-15(22)11-19(13)30-17-6-8-27(9-7-17)21-26-25-14(2)28(21)16-4-5-20(29-3)24-12-16/h4-5,10-12,17H,6-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase assay |
Bioorg Med Chem Lett 20: 516-20 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.097
BindingDB Entry DOI: 10.7270/Q2MS3SWF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... |
Bioorg Med Chem 24: 3513-20 (2016)
Article DOI: 10.1016/j.bmc.2016.05.062
BindingDB Entry DOI: 10.7270/Q2WH2RXT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077141

(CHEMBL3416758)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)C(N)C2(CCCCC2)SSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)[C@@H](C)O |r| Show InChI InChI=1S/C57H78N12O13S2/c1-32(70)46-54(79)65-42(30-45(59)72)51(76)67-43(56(81)69-26-10-14-44(69)53(78)62-38(13-9-25-58)49(74)63-39(48(61)73)27-34-15-19-36(71)20-16-34)31-83-84-57(23-7-4-8-24-57)47(60)55(80)66-40(29-35-17-21-37(82-2)22-18-35)50(75)64-41(52(77)68-46)28-33-11-5-3-6-12-33/h3,5-6,11-12,15-22,32,38-44,46-47,70-71H,4,7-10,13-14,23-31,58,60H2,1-2H3,(H2,59,72)(H2,61,73)(H,62,78)(H,63,74)(H,64,75)(H,65,79)(H,66,80)(H,67,76)(H,68,77)/t32-,38+,39+,40+,41+,42+,43+,44+,46+,47?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Competitive binding to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells incubated for 1 hr at RT followed by 4 hrs at 4 degC ... |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM86210
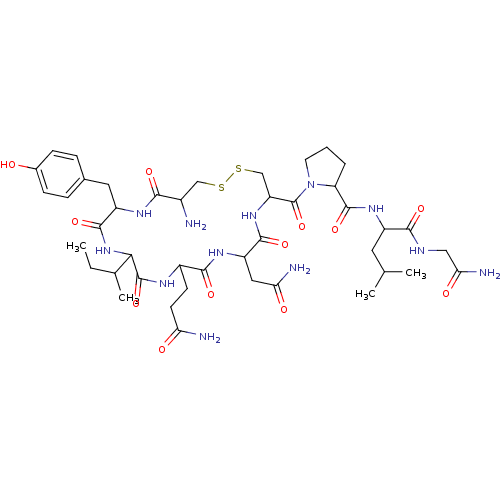
(CAS_50-56-6 | NSC_439302 | Oxytocin)Show SMILES CCC(C)C1NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(N)CSSCC(NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 253-61 (2003)
Article DOI: 10.1124/jpet.103.049395
BindingDB Entry DOI: 10.7270/Q2MC8XKT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326716
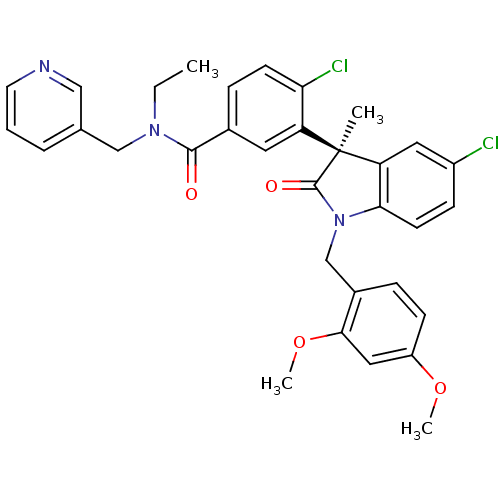
((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...)Show SMILES CCN(Cc1cccnc1)C(=O)c1ccc(Cl)c(c1)[C@]1(C)C(=O)N(Cc2ccc(OC)cc2OC)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C33H31Cl2N3O4/c1-5-37(19-21-7-6-14-36-18-21)31(39)22-9-12-28(35)26(15-22)33(2)27-16-24(34)10-13-29(27)38(32(33)40)20-23-8-11-25(41-3)17-30(23)42-4/h6-18H,5,19-20H2,1-4H3/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384809

(CHEMBL2037493)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O3/c1-18(2)14-24-27(34)31-25(23-15-20-8-4-5-9-21(20)16-23)28(35)33(24)26(22-11-10-19(3)30-17-22)29(36)32-12-6-7-13-32/h4-5,8-11,17-18,23-26H,6-7,12-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384804
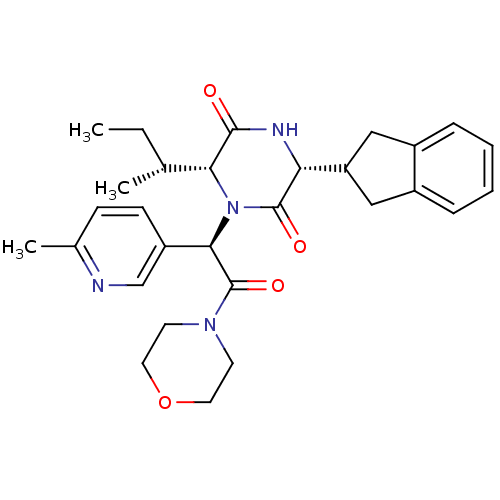
(CHEMBL2037500)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219760
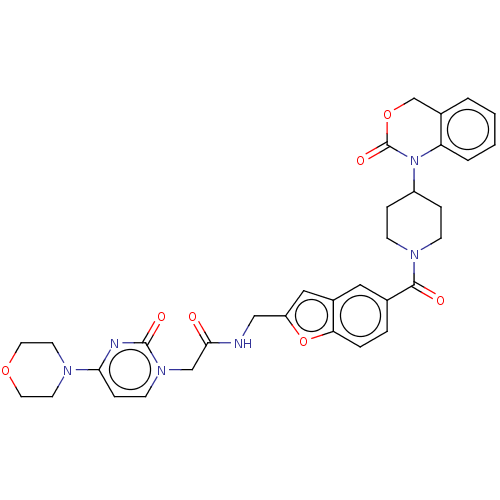
(CHEMBL286895)Show SMILES O=C(Cn1ccc(nc1=O)N1CCOCC1)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C33H34N6O7/c40-30(20-38-12-9-29(35-32(38)42)36-13-15-44-16-14-36)34-19-26-18-24-17-22(5-6-28(24)46-26)31(41)37-10-7-25(8-11-37)39-27-4-2-1-3-23(27)21-45-33(39)43/h1-6,9,12,17-18,25H,7-8,10-11,13-16,19-21H2,(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523555
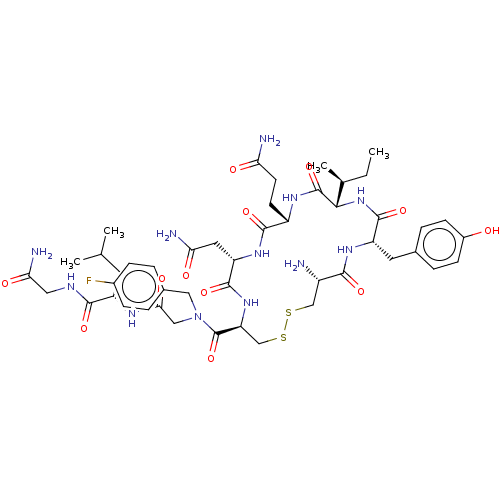
(CHEMBL4474284)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1 |r| Show InChI InChI=1S/C47H67FN12O12S2/c1-5-25(4)40-46(71)55-31(14-15-36(50)62)43(68)57-34(18-37(51)63)44(69)58-35(23-74-73-22-30(49)41(66)56-33(45(70)59-40)17-26-8-12-29(61)13-9-26)47(72)60(20-27-6-10-28(48)11-7-27)21-39(65)54-32(16-24(2)3)42(67)53-19-38(52)64/h6-13,24-25,30-35,40,61H,5,14-23,49H2,1-4H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,71)(H,56,66)(H,57,68)(H,58,69)(H,59,70)/t25-,30-,31-,32-,33-,34-,35-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103477
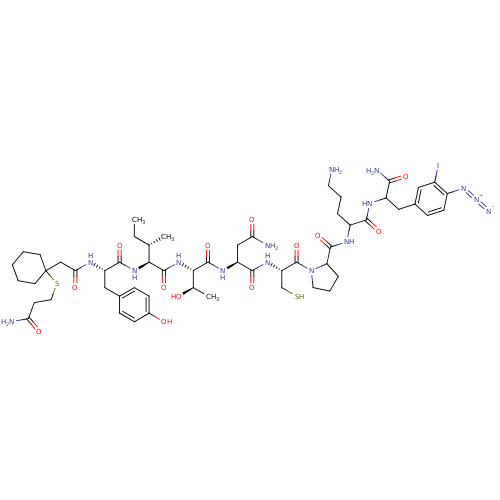
(CHEMBL386180 | Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C56H82IN15O13S2/c1-4-30(2)46(68-51(81)39(25-32-12-15-34(74)16-13-32)63-45(77)28-56(19-6-5-7-20-56)87-23-18-43(59)75)53(83)69-47(31(3)73)54(84)66-40(27-44(60)76)50(80)67-41(29-86)55(85)72-22-9-11-42(72)52(82)64-37(10-8-21-58)49(79)65-38(48(61)78)26-33-14-17-36(70-71-62)35(57)24-33/h12-17,24,30-31,37-42,46-47,73-74,86H,4-11,18-23,25-29,58H2,1-3H3,(H2,59,75)(H2,60,76)(H2,61,78)(H,63,77)(H,64,82)(H,65,79)(H,66,84)(H,67,80)(H,68,81)(H,69,83)/t30-,31+,37?,38?,39-,40-,41-,42?,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity towards OT receptor in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50077035

(1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...)Show SMILES FC(F)(F)COc1cc(ccc1CC(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12)N1CCOCC1 Show InChI InChI=1S/C27H30F3N3O5/c28-27(29,30)18-38-24-16-22(31-11-13-36-14-12-31)6-5-19(24)15-25(34)32-9-7-21(8-10-32)33-23-4-2-1-3-20(23)17-37-26(33)35/h1-6,16,21H,7-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat uterine oxytocin receptor (rOTr) |
Bioorg Med Chem Lett 9: 1311-6 (1999)
BindingDB Entry DOI: 10.7270/Q23N22K9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326714

((3R,6R)-6-sec-butyl-3-(2,3-dihydro-1H-inden-2-yl)-...)Show SMILES CC[C@H](C)[C@@]1(C)N([C@@H](C(=O)N2CCOCC2)c2coc(C)n2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O5/c1-5-17(2)28(4)27(35)30-23(21-14-19-8-6-7-9-20(19)15-21)25(33)32(28)24(22-16-37-18(3)29-22)26(34)31-10-12-36-13-11-31/h6-9,16-17,21,23-24H,5,10-15H2,1-4H3,(H,30,35)/t17-,23+,24+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50038604
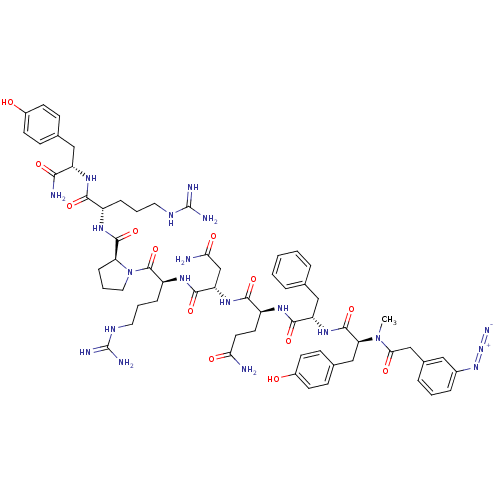
(3-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-11-5-12-39(29-38)79-80-70)49(32-37-18-22-41(84)23-19-37)59(94)78-46(31-35-9-3-2-4-10-35)56(91)73-43(24-25-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(14-7-27-72-62(68)69)60(95)82-28-8-15-48(82)58(93)74-42(13-6-26-71-61(66)67)54(89)76-45(53(65)88)30-36-16-20-40(83)21-17-36/h2-5,9-12,16-23,29,42-49,83-84H,6-8,13-15,24-28,30-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at OT receptor of rat mamary glands |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326722
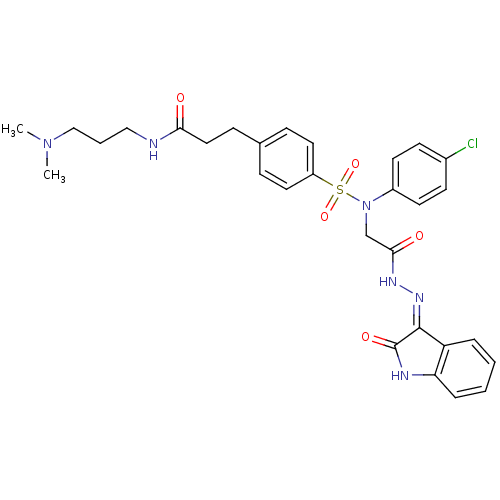
((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data