 Found 10 hits of ec50 for UniProtKB: Q9UBL9
Found 10 hits of ec50 for UniProtKB: Q9UBL9 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50370141
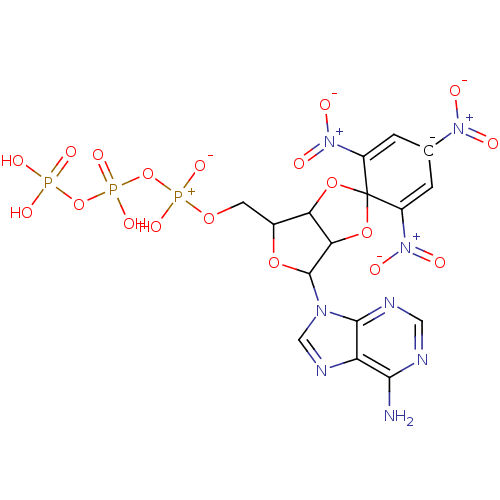
(TNP-ATP)Show SMILES Nc1ncnc2n(cnc12)C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C2OC3(OC12)C(=C[C-](C=C3[N+]([O-])=O)[N+]([O-])=O)[N+]([O-])=O |c:36,39,(6.91,.64,;6.89,-.9,;5.56,-1.66,;5.55,-3.21,;6.88,-3.97,;8.21,-3.22,;9.67,-3.7,;10.58,-2.46,;9.68,-1.21,;8.22,-1.68,;10.14,-5.17,;9.23,-6.42,;10.13,-7.66,;9.36,-9,;7.82,-9.01,;7.06,-10.34,;5.72,-9.57,;8.15,-11.43,;5.96,-11.43,;4.42,-11.44,;4.39,-9.89,;3.66,-12.79,;2.92,-11.07,;1.38,-11.1,;.93,-9.62,;-.11,-11.54,;1.4,-12.64,;11.6,-7.19,;13.07,-7.68,;13.97,-6.42,;13.07,-5.18,;11.6,-5.65,;14.73,-7.75,;16.26,-7.76,;17.03,-6.44,;16.26,-5.11,;14.73,-5.1,;13.96,-3.76,;14.73,-2.42,;12.42,-3.75,;18.57,-6.45,;19.34,-5.11,;19.34,-7.79,;13.95,-9.09,;12.41,-9.08,;14.72,-10.43,)| Show InChI InChI=1S/C16H16N8O19P3/c17-13-10-14(19-4-18-13)21(5-20-10)15-12-11(7(39-15)3-38-45(34,35)43-46(36,37)42-44(31,32)33)40-16(41-12)8(23(27)28)1-6(22(25)26)2-9(16)24(29)30/h1-2,4-5,7,11-12,15H,3H2,(H,34,35)(H,36,37)(H2,17,18,19)(H2,31,32,33)/q-1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against recombinant human receptor P2X purinoceptor 2 (P2X2) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50118219
(Bz-ATP | CHEMBL339386)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1OC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H24N5O14P3/c24-20-17-21(26-11-25-20)28(12-27-17)22-19(40-23(30)15-8-6-14(7-9-15)13-4-2-1-3-5-13)18(29)16(39-22)10-38-44(34,35)42-45(36,37)41-43(31,32)33/h1-9,11-12,16,18-19,22,29H,10H2,(H,34,35)(H,36,37)(H2,24,25,26)(H2,31,32,33)/t16-,18-,19-,22-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against recombinant human receptor P2X purinoceptor 2 (P2X2 ) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598316
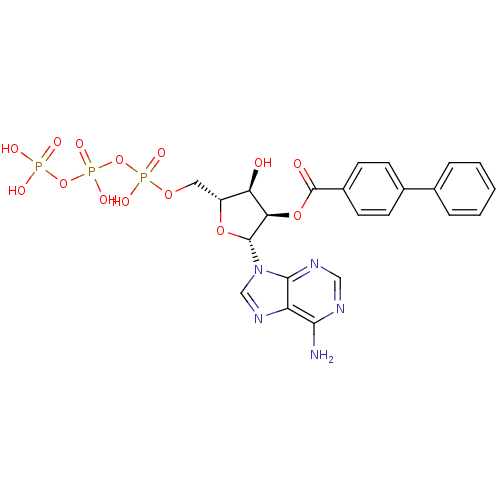
(CHEMBL5184613) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.27E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598316

(CHEMBL5184613) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598316

(CHEMBL5184613) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50118220
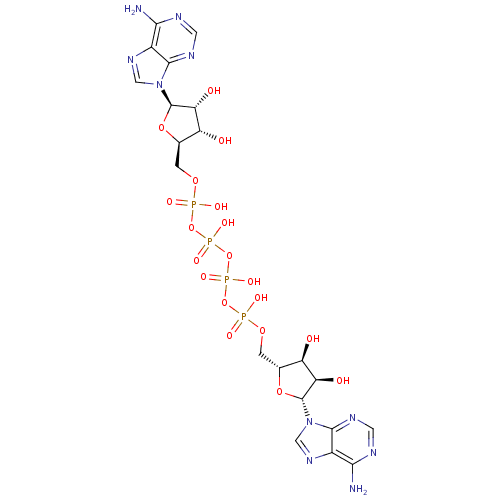
((ppA)2 | A(5')p4(5')A | CHEMBL339385 | P(1),P(4)-b...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H28N10O19P4/c21-15-9-17(25-3-23-15)29(5-27-9)19-13(33)11(31)7(45-19)1-43-50(35,36)47-52(39,40)49-53(41,42)48-51(37,38)44-2-8-12(32)14(34)20(46-8)30-6-28-10-16(22)24-4-26-18(10)30/h3-8,11-14,19-20,31-34H,1-2H2,(H,35,36)(H,37,38)(H,39,40)(H,41,42)(H2,21,23,25)(H2,22,24,26)/t7-,8-,11-,12-,13-,14-,19-,20-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against recombinant human receptor P2X purinoceptor 2 (P2X2 ) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50118216
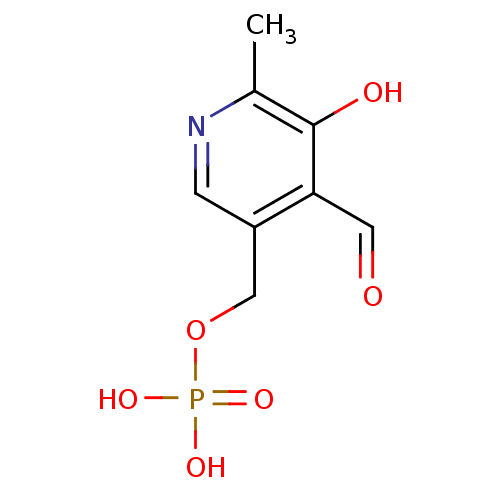
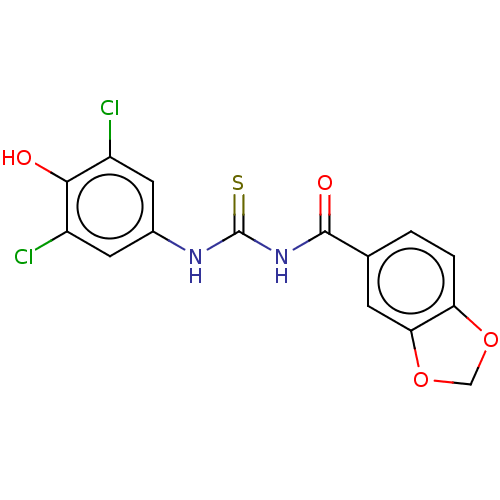
((4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl di...)Show InChI InChI=1S/C8H10NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2-3,11H,4H2,1H3,(H2,12,13,14) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against recombinant human receptor P2X purinoceptor 2 (P2X2 ) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50368125

(ADENOSINE DIPHOSPHATE | ADP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against recombinant human receptor P2X purinoceptor 2 (P2X2 ) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50594505
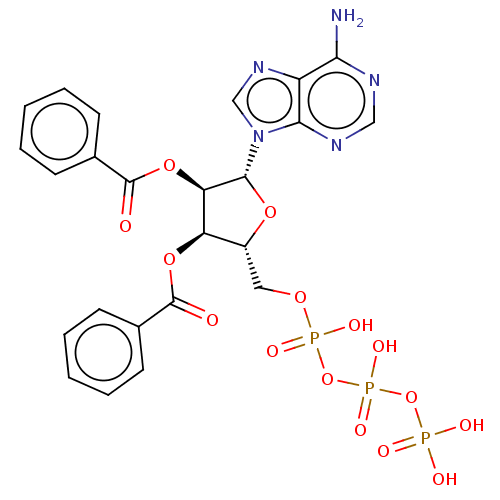
(CHEMBL5177316)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00812
BindingDB Entry DOI: 10.7270/Q2XS60DK |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50594506
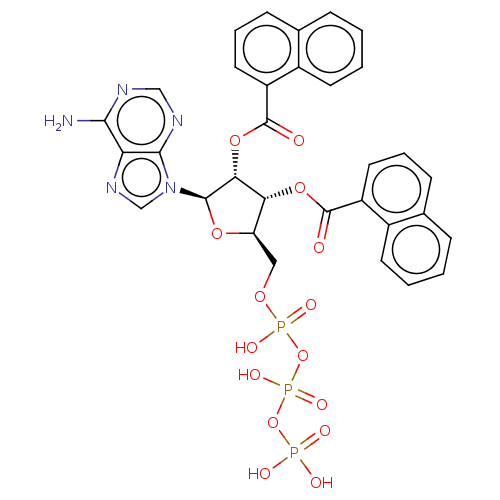
(CHEMBL5183218)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](OC(=O)c2cccc3ccccc23)[C@H]1OC(=O)c1cccc2ccccc12 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00812
BindingDB Entry DOI: 10.7270/Q2XS60DK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data