 Found 50 hits of ic50 for UniProtKB: P30938
Found 50 hits of ic50 for UniProtKB: P30938 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476140
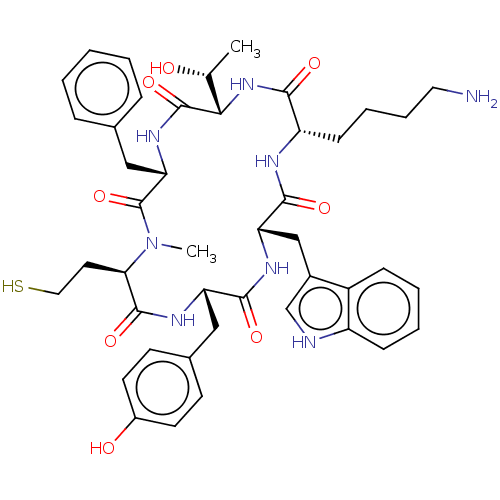
(CHEMBL373632)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCS)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C44H56N8O8S/c1-26(53)38-43(59)50-36(23-27-10-4-3-5-11-27)44(60)52(2)37(19-21-61)42(58)49-34(22-28-15-17-30(54)18-16-28)40(56)48-35(24-29-25-46-32-13-7-6-12-31(29)32)41(57)47-33(39(55)51-38)14-8-9-20-45/h3-7,10-13,15-18,25-26,33-38,46,53-54,61H,8-9,14,19-24,45H2,1-2H3,(H,47,57)(H,48,56)(H,49,58)(H,50,59)(H,51,55)/t26-,33+,34+,35+,36+,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476137

(CHEMBL222751)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2ccccc2)N(C)C(=O)[C@H](CCS)NC1=O)[C@@H](C)O Show InChI InChI=1S/C44H56N8O8S/c1-26(53)38-43(59)48-34(19-21-61)44(60)52(2)37(23-27-10-4-3-5-11-27)42(58)50-35(22-28-15-17-30(54)18-16-28)40(56)49-36(24-29-25-46-32-13-7-6-12-31(29)32)41(57)47-33(39(55)51-38)14-8-9-20-45/h3-7,10-13,15-18,25-26,33-38,46,53-54,61H,8-9,14,19-24,45H2,1-2H3,(H,47,57)(H,48,59)(H,49,56)(H,50,58)(H,51,55)/t26-,33+,34+,35+,36+,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its inhibitory effect against somatostatin receptor in rat cortex membrane using [125I]Tyr3-octreotide as the radioligand |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20G3NBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50214421
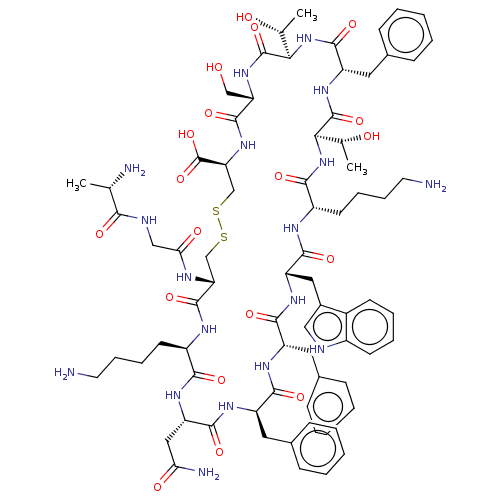
(SOMATOSTATIN RECEPTOR LIGAND)Show SMILES [H][C@@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@]([H])(NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55-,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its inhibitory effect against somatostatin receptor in rat cortex membrane using [125I]Tyr3-octreotide as the radioligand |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20G3NBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476135

(CHEMBL374316)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCS)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C45H58N8O7S/c1-27(2)39-44(59)51-37(24-28-11-5-4-6-12-28)45(60)53(3)38(20-22-61)43(58)50-35(23-29-16-18-31(54)19-17-29)41(56)49-36(25-30-26-47-33-14-8-7-13-32(30)33)42(57)48-34(40(55)52-39)15-9-10-21-46/h4-8,11-14,16-19,26-27,34-39,47,54,61H,9-10,15,20-25,46H2,1-3H3,(H,48,57)(H,49,56)(H,50,58)(H,51,59)(H,52,55)/t34-,35-,36-,37-,38+,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50272772

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES C[C@@H](O)[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059093

(2-{[10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propio...)Show SMILES C[C@@H](O)[C@@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@H]([C@@H](C)O)C(=O)N1)C(O)=O Show InChI InChI=1S/C49H64N10O11S2/c1-27(60)40-48(68)57-39(47(67)59-41(28(2)61)49(69)70)26-72-71-25-38(56-42(62)33(51)21-29-13-5-3-6-14-29)46(66)54-36(22-30-15-7-4-8-16-30)44(64)55-37(23-31-24-52-34-18-10-9-17-32(31)34)45(65)53-35(43(63)58-40)19-11-12-20-50/h3-10,13-18,24,27-28,33,35-41,52,60-61H,11-12,19-23,25-26,50-51H2,1-2H3,(H,53,65)(H,54,66)(H,55,64)(H,56,62)(H,57,68)(H,58,63)(H,59,67)(H,69,70)/t27-,28-,33+,35+,36+,37-,38+,39-,40-,41-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of rSSTR5 |
J Med Chem 40: 2252-8 (1997)
Article DOI: 10.1021/jm960851a
BindingDB Entry DOI: 10.7270/Q2BC3XPS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059091
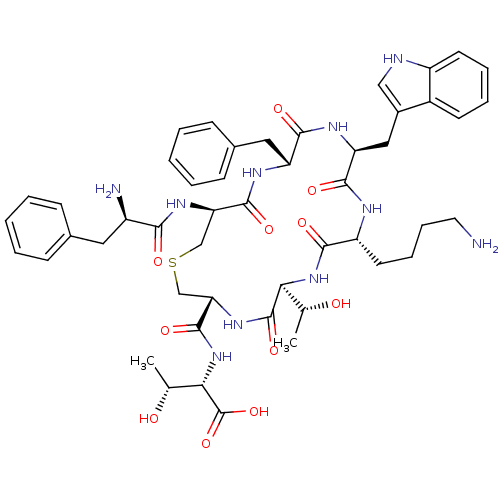
(2-{[9-(4-Amino-butyl)-18-(2-amino-3-phenyl-propion...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CSC[C@@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1)C(O)=O Show InChI InChI=1S/C49H64N10O11S/c1-27(60)40-48(68)57-39(47(67)59-41(28(2)61)49(69)70)26-71-25-38(56-42(62)33(51)21-29-13-5-3-6-14-29)46(66)54-36(22-30-15-7-4-8-16-30)44(64)55-37(23-31-24-52-34-18-10-9-17-32(31)34)45(65)53-35(43(63)58-40)19-11-12-20-50/h3-10,13-18,24,27-28,33,35-41,52,60-61H,11-12,19-23,25-26,50-51H2,1-2H3,(H,53,65)(H,54,66)(H,55,64)(H,56,62)(H,57,68)(H,58,63)(H,59,67)(H,69,70)/t27-,28-,33-,35-,36-,37+,38-,39+,40+,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of rSSTR5 |
J Med Chem 40: 2252-8 (1997)
Article DOI: 10.1021/jm960851a
BindingDB Entry DOI: 10.7270/Q2BC3XPS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059091

(2-{[9-(4-Amino-butyl)-18-(2-amino-3-phenyl-propion...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CSC[C@@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1)C(O)=O Show InChI InChI=1S/C49H64N10O11S/c1-27(60)40-48(68)57-39(47(67)59-41(28(2)61)49(69)70)26-71-25-38(56-42(62)33(51)21-29-13-5-3-6-14-29)46(66)54-36(22-30-15-7-4-8-16-30)44(64)55-37(23-31-24-52-34-18-10-9-17-32(31)34)45(65)53-35(43(63)58-40)19-11-12-20-50/h3-10,13-18,24,27-28,33,35-41,52,60-61H,11-12,19-23,25-26,50-51H2,1-2H3,(H,53,65)(H,54,66)(H,55,64)(H,56,62)(H,57,68)(H,58,63)(H,59,67)(H,69,70)/t27-,28-,33-,35-,36-,37+,38-,39+,40+,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476143

(CHEMBL376001)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2ccccc2)N(C)C(=O)[C@H](CCS)NC1=O Show InChI InChI=1S/C45H58N8O7S/c1-27(2)39-44(59)49-35(20-22-61)45(60)53(3)38(24-28-11-5-4-6-12-28)43(58)51-36(23-29-16-18-31(54)19-17-29)41(56)50-37(25-30-26-47-33-14-8-7-13-32(30)33)42(57)48-34(40(55)52-39)15-9-10-21-46/h4-8,11-14,16-19,26-27,34-39,47,54,61H,9-10,15,20-25,46H2,1-3H3,(H,48,57)(H,49,59)(H,50,56)(H,51,58)(H,52,55)/t34-,35-,36-,37-,38+,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059089
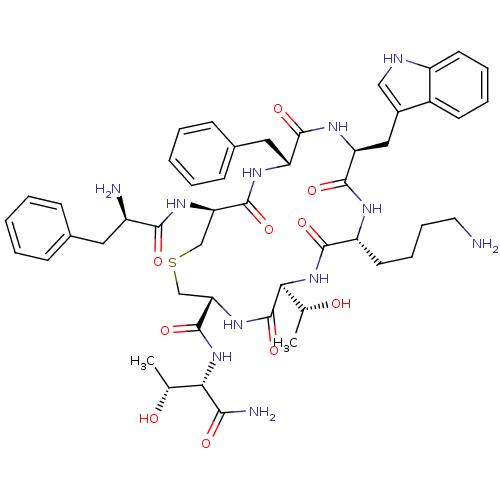
(9-(4-Amino-butyl)-18-(2-amino-3-phenyl-propionylam...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CSC[C@@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1)C(N)=O Show InChI InChI=1S/C49H65N11O10S/c1-27(61)40(42(52)63)59-48(69)39-26-71-25-38(57-43(64)33(51)21-29-13-5-3-6-14-29)47(68)55-36(22-30-15-7-4-8-16-30)45(66)56-37(23-31-24-53-34-18-10-9-17-32(31)34)46(67)54-35(19-11-12-20-50)44(65)60-41(28(2)62)49(70)58-39/h3-10,13-18,24,27-28,33,35-41,53,61-62H,11-12,19-23,25-26,50-51H2,1-2H3,(H2,52,63)(H,54,67)(H,55,68)(H,56,66)(H,57,64)(H,58,70)(H,59,69)(H,60,65)/t27-,28-,33-,35-,36-,37+,38-,39+,40+,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059089

(9-(4-Amino-butyl)-18-(2-amino-3-phenyl-propionylam...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CSC[C@@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1)C(N)=O Show InChI InChI=1S/C49H65N11O10S/c1-27(61)40(42(52)63)59-48(69)39-26-71-25-38(57-43(64)33(51)21-29-13-5-3-6-14-29)47(68)55-36(22-30-15-7-4-8-16-30)45(66)56-37(23-31-24-53-34-18-10-9-17-32(31)34)46(67)54-35(19-11-12-20-50)44(65)60-41(28(2)62)49(70)58-39/h3-10,13-18,24,27-28,33,35-41,53,61-62H,11-12,19-23,25-26,50-51H2,1-2H3,(H2,52,63)(H,54,67)(H,55,68)(H,56,66)(H,57,64)(H,58,70)(H,59,69)(H,60,65)/t27-,28-,33-,35-,36-,37+,38-,39+,40+,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of rSSTR5 |
J Med Chem 40: 2252-8 (1997)
Article DOI: 10.1021/jm960851a
BindingDB Entry DOI: 10.7270/Q2BC3XPS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059092
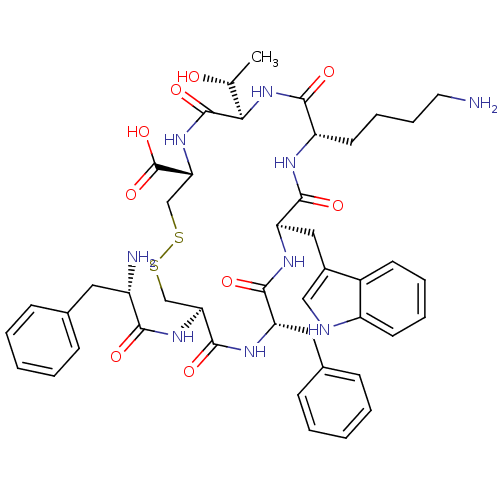
((4S,7R,10S,13R,16S,19R)-10-(4-Amino-butyl)-19-((S)...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(O)=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C45H57N9O9S2/c1-26(55)38-44(61)53-37(45(62)63)25-65-64-24-36(52-39(56)31(47)20-27-12-4-2-5-13-27)43(60)50-34(21-28-14-6-3-7-15-28)41(58)51-35(22-29-23-48-32-17-9-8-16-30(29)32)42(59)49-33(40(57)54-38)18-10-11-19-46/h2-9,12-17,23,26,31,33-38,48,55H,10-11,18-22,24-25,46-47H2,1H3,(H,49,59)(H,50,60)(H,51,58)(H,52,56)(H,53,61)(H,54,57)(H,62,63)/t26-,31+,33+,34+,35-,36+,37-,38-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of rSSTR5 |
J Med Chem 40: 2252-8 (1997)
Article DOI: 10.1021/jm960851a
BindingDB Entry DOI: 10.7270/Q2BC3XPS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059092

((4S,7R,10S,13R,16S,19R)-10-(4-Amino-butyl)-19-((S)...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(O)=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C45H57N9O9S2/c1-26(55)38-44(61)53-37(45(62)63)25-65-64-24-36(52-39(56)31(47)20-27-12-4-2-5-13-27)43(60)50-34(21-28-14-6-3-7-15-28)41(58)51-35(22-29-23-48-32-17-9-8-16-30(29)32)42(59)49-33(40(57)54-38)18-10-11-19-46/h2-9,12-17,23,26,31,33-38,48,55H,10-11,18-22,24-25,46-47H2,1H3,(H,49,59)(H,50,60)(H,51,58)(H,52,56)(H,53,61)(H,54,57)(H,62,63)/t26-,31+,33+,34+,35-,36+,37-,38-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476144
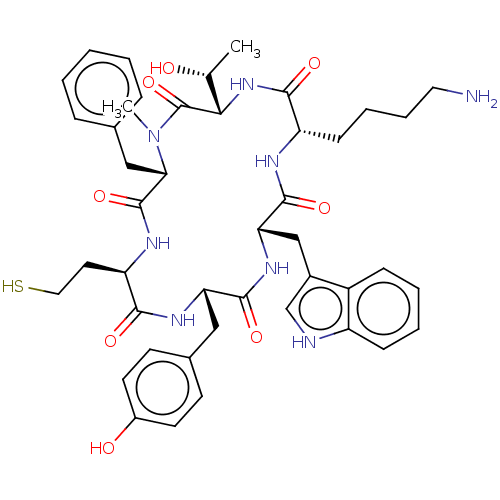
(CHEMBL434761)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCS)NC(=O)[C@H](Cc2ccccc2)N(C)C1=O)[C@@H](C)O Show InChI InChI=1S/C44H56N8O8S/c1-26(53)38-44(60)52(2)37(23-27-10-4-3-5-11-27)43(59)48-34(19-21-61)39(55)49-35(22-28-15-17-30(54)18-16-28)41(57)50-36(24-29-25-46-32-13-7-6-12-31(29)32)42(58)47-33(40(56)51-38)14-8-9-20-45/h3-7,10-13,15-18,25-26,33-38,46,53-54,61H,8-9,14,19-24,45H2,1-2H3,(H,47,58)(H,48,59)(H,49,55)(H,50,57)(H,51,56)/t26-,33+,34-,35+,36+,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | CHEMBL5282589
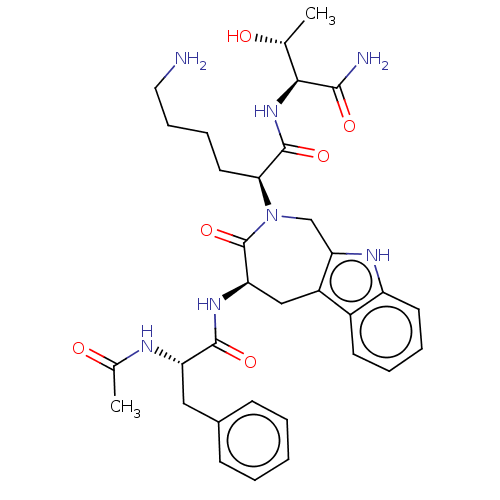
| UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059087
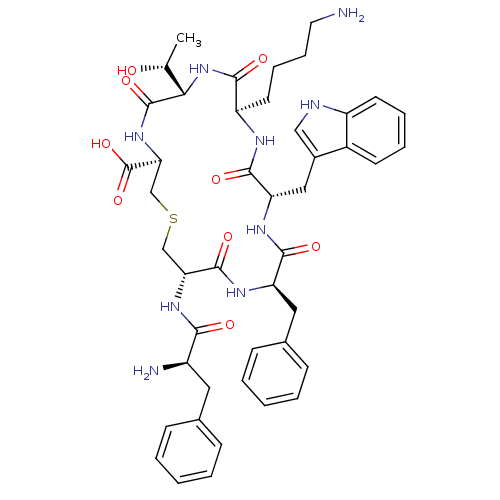
((3R,6S,9R,12S,15R,18S)-9-(4-Amino-butyl)-18-((R)-2...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CSC[C@H](NC1=O)C(O)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C45H57N9O9S/c1-26(55)38-44(61)53-37(45(62)63)25-64-24-36(52-39(56)31(47)20-27-12-4-2-5-13-27)43(60)50-34(21-28-14-6-3-7-15-28)41(58)51-35(22-29-23-48-32-17-9-8-16-30(29)32)42(59)49-33(40(57)54-38)18-10-11-19-46/h2-9,12-17,23,26,31,33-38,48,55H,10-11,18-22,24-25,46-47H2,1H3,(H,49,59)(H,50,60)(H,51,58)(H,52,56)(H,53,61)(H,54,57)(H,62,63)/t26-,31-,33-,34-,35+,36-,37+,38+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059087

((3R,6S,9R,12S,15R,18S)-9-(4-Amino-butyl)-18-((R)-2...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CSC[C@H](NC1=O)C(O)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C45H57N9O9S/c1-26(55)38-44(61)53-37(45(62)63)25-64-24-36(52-39(56)31(47)20-27-12-4-2-5-13-27)43(60)50-34(21-28-14-6-3-7-15-28)41(58)51-35(22-29-23-48-32-17-9-8-16-30(29)32)42(59)49-33(40(57)54-38)18-10-11-19-46/h2-9,12-17,23,26,31,33-38,48,55H,10-11,18-22,24-25,46-47H2,1H3,(H,49,59)(H,50,60)(H,51,58)(H,52,56)(H,53,61)(H,54,57)(H,62,63)/t26-,31-,33-,34-,35+,36-,37+,38+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of rSSTR5 |
J Med Chem 40: 2252-8 (1997)
Article DOI: 10.1021/jm960851a
BindingDB Entry DOI: 10.7270/Q2BC3XPS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50059088
((3S,6R,9S,12R,15S,18R)-18-Amino-9-(4-amino-butyl)-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](N)CSC[C@@H](NC1=O)C(O)=O Show InChI InChI=1S/C36H48N8O8S/c1-20(45)30-35(50)43-29(36(51)52)19-53-18-24(38)31(46)41-27(15-21-9-3-2-4-10-21)33(48)42-28(16-22-17-39-25-12-6-5-11-23(22)25)34(49)40-26(32(47)44-30)13-7-8-14-37/h2-6,9-12,17,20,24,26-30,39,45H,7-8,13-16,18-19,37-38H2,1H3,(H,40,49)(H,41,46)(H,42,48)(H,43,50)(H,44,47)(H,51,52)/t20-,24+,26+,27+,28-,29-,30-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Tested for inhibition of radioligand binding to cloned somatostatin receptor rSSTR5 |
J Med Chem 40: 2241-51 (1997)
Article DOI: 10.1021/jm960850i
BindingDB Entry DOI: 10.7270/Q2125TBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020202
(CHEMBL2369466 | D-Tic-c(Cys-Tyr-D-Trp-Lys-Thr-Pen-...)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H]1Cc2ccccc2CN1)[C@@H](C)O Show InChI InChI=1S/C52H69N11O11S2/c1-27(64)41(44(54)67)61-51(74)43-52(3,4)76-75-26-40(60-46(69)37-22-30-11-5-6-12-31(30)24-56-37)49(72)58-38(21-29-16-18-33(66)19-17-29)47(70)59-39(23-32-25-55-35-14-8-7-13-34(32)35)48(71)57-36(15-9-10-20-53)45(68)62-42(28(2)65)50(73)63-43/h5-8,11-14,16-19,25,27-28,36-43,55-56,64-66H,9-10,15,20-24,26,53H2,1-4H3,(H2,54,67)(H,57,71)(H,58,72)(H,59,70)(H,60,69)(H,61,74)(H,62,68)(H,63,73)/t27-,28-,36+,37-,38-,39+,40+,41+,42+,43-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 949 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020209
(CHEMBL410090 | CTP)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C51H69N11O11S2/c1-27(63)40(43(54)66)60-50(73)42-51(3,4)75-74-26-39(59-44(67)34(53)22-29-12-6-5-7-13-29)48(71)57-37(23-30-17-19-32(65)20-18-30)46(69)58-38(24-31-25-55-35-15-9-8-14-33(31)35)47(70)56-36(16-10-11-21-52)45(68)61-41(28(2)64)49(72)62-42/h5-9,12-15,17-20,25,27-28,34,36-42,55,63-65H,10-11,16,21-24,26,52-53H2,1-4H3,(H2,54,66)(H,56,70)(H,57,71)(H,58,69)(H,59,67)(H,60,73)(H,61,68)(H,62,72)/t27-,28-,34+,36+,37+,38+,39+,40+,41+,42-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020205

(CHEMBL216354 | D-Tic-c(Cys-Tyr-D-Trp-Lys-Thr-Pen-)...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C51H67N11O11S2/c1-27(64)41-49(72)62-42(50(73)59-39(25-63)43(53)66)51(2,3)75-74-26-40(60-45(68)36-21-29-10-4-5-11-30(29)23-55-36)48(71)57-37(20-28-15-17-32(65)18-16-28)46(69)58-38(22-31-24-54-34-13-7-6-12-33(31)34)47(70)56-35(44(67)61-41)14-8-9-19-52/h4-7,10-13,15-18,24,27,35-42,54-55,63-65H,8-9,14,19-23,25-26,52H2,1-3H3,(H2,53,66)(H,56,70)(H,57,71)(H,58,69)(H,59,73)(H,60,68)(H,61,67)(H,62,72)/t27-,35+,36-,37+,38+,39+,40+,41+,42-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020213
(CHEMBL264082 | D-Trpc(-Cys-Tyr-D-Trp-Lys-Thr-Pen-)...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C53H70N12O11S2/c1-27(66)42(45(56)69)63-52(76)44-53(3,4)78-77-26-41(62-46(70)35(55)22-30-24-57-36-13-7-5-11-33(30)36)50(74)60-39(21-29-16-18-32(68)19-17-29)48(72)61-40(23-31-25-58-37-14-8-6-12-34(31)37)49(73)59-38(15-9-10-20-54)47(71)64-43(28(2)67)51(75)65-44/h5-8,11-14,16-19,24-25,27-28,35,38-44,57-58,66-68H,9-10,15,20-23,26,54-55H2,1-4H3,(H2,56,69)(H,59,73)(H,60,74)(H,61,72)(H,62,70)(H,63,76)(H,64,71)(H,65,75)/t27-,28-,35+,38+,39+,40+,41+,42+,43+,44-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020211

(CHEMBL216353 | D-Phec(-Cys-Tyr-D-Trp-Lys-Thr-Pen-)...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C51H68N12O11S2/c1-27(64)41-49(73)63-42(50(74)58-36(43(55)67)24-40(54)66)51(2,3)76-75-26-39(61-44(68)33(53)21-28-11-5-4-6-12-28)48(72)59-37(22-29-16-18-31(65)19-17-29)46(70)60-38(23-30-25-56-34-14-8-7-13-32(30)34)47(71)57-35(45(69)62-41)15-9-10-20-52/h4-8,11-14,16-19,25,27,33,35-39,41-42,56,64-65H,9-10,15,20-24,26,52-53H2,1-3H3,(H2,54,66)(H2,55,67)(H,57,71)(H,58,74)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,63,73)/t27-,33-,35+,36+,37+,38+,39+,41+,42-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020216

(CHEMBL413831 | D-Phec(-Cys-Tyr-D-Trp-Lys-Thr-Pen)-...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C50H67N11O11S2/c1-27(63)40-48(71)61-41(49(72)58-38(25-62)42(53)65)50(2,3)74-73-26-39(59-43(66)33(52)21-28-11-5-4-6-12-28)47(70)56-36(22-29-16-18-31(64)19-17-29)45(68)57-37(23-30-24-54-34-14-8-7-13-32(30)34)46(69)55-35(44(67)60-40)15-9-10-20-51/h4-8,11-14,16-19,24,27,33,35-41,54,62-64H,9-10,15,20-23,25-26,51-52H2,1-3H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,72)(H,59,66)(H,60,67)(H,61,71)/t27-,33-,35+,36+,37+,38+,39+,40+,41-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020198
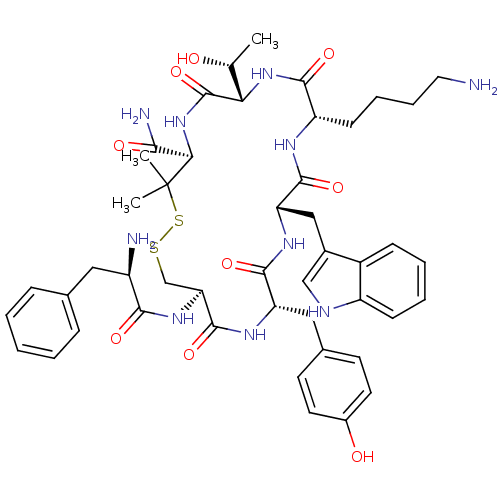
(CHEMBL308471 | D-Phec(-Cys-Tyr-D-Trp-Lys-Thr-Pen-)...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C47H62N10O9S2/c1-26(58)38-46(66)57-39(40(50)60)47(2,3)68-67-25-37(55-41(61)32(49)21-27-11-5-4-6-12-27)45(65)53-35(22-28-16-18-30(59)19-17-28)43(63)54-36(23-29-24-51-33-14-8-7-13-31(29)33)44(64)52-34(42(62)56-38)15-9-10-20-48/h4-8,11-14,16-19,24,26,32,34-39,51,58-59H,9-10,15,20-23,25,48-49H2,1-3H3,(H2,50,60)(H,52,64)(H,53,65)(H,54,63)(H,55,61)(H,56,62)(H,57,66)/t26-,32-,34+,35+,36+,37+,38+,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020200
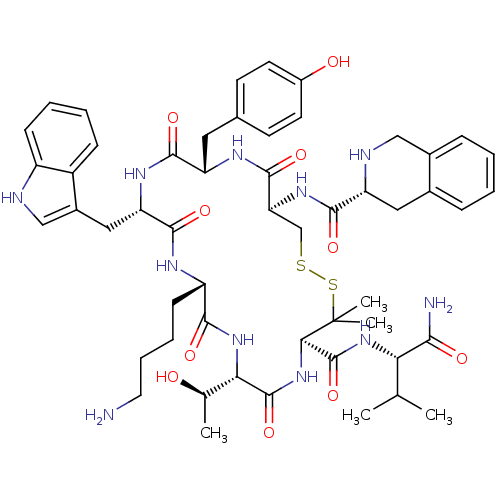
(CHEMBL405672 | D-Ticc(-Cys-Tyr-D-Typ-Lys-Thr-Pen-)...)Show SMILES CC(C)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H]1Cc2ccccc2CN1)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C53H71N11O10S2/c1-28(2)42(45(55)67)62-52(74)44-53(4,5)76-75-27-41(61-47(69)38-23-31-12-6-7-13-32(31)25-57-38)50(72)59-39(22-30-17-19-34(66)20-18-30)48(70)60-40(24-33-26-56-36-15-9-8-14-35(33)36)49(71)58-37(16-10-11-21-54)46(68)63-43(29(3)65)51(73)64-44/h6-9,12-15,17-20,26,28-29,37-44,56-57,65-66H,10-11,16,21-25,27,54H2,1-5H3,(H2,55,67)(H,58,71)(H,59,72)(H,60,70)(H,61,69)(H,62,74)(H,63,68)(H,64,73)/t29-,37+,38-,39+,40+,41+,42+,43+,44-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020214
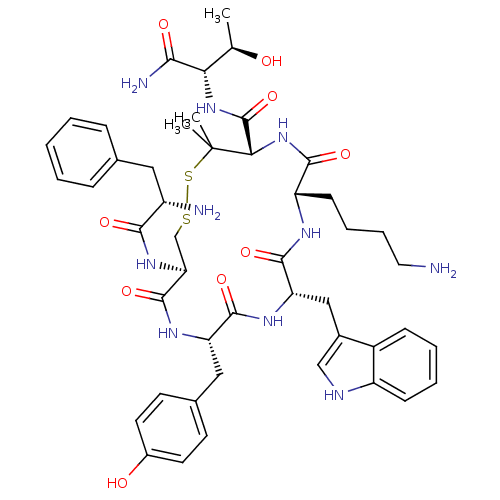
(CHEMBL302596 | D-Phec(-Cys-Tyr-D-Trp-Lys-D-Pen-)Th...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H62N10O9S2/c1-26(58)38(40(50)60)56-46(66)39-47(2,3)68-67-25-37(55-41(61)32(49)21-27-11-5-4-6-12-27)45(65)53-35(22-28-16-18-30(59)19-17-28)43(63)54-36(23-29-24-51-33-14-8-7-13-31(29)33)44(64)52-34(42(62)57-39)15-9-10-20-48/h4-8,11-14,16-19,24,26,32,34-39,51,58-59H,9-10,15,20-23,25,48-49H2,1-3H3,(H2,50,60)(H,52,64)(H,53,65)(H,54,63)(H,55,61)(H,56,66)(H,57,62)/t26-,32+,34+,35+,36+,37+,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
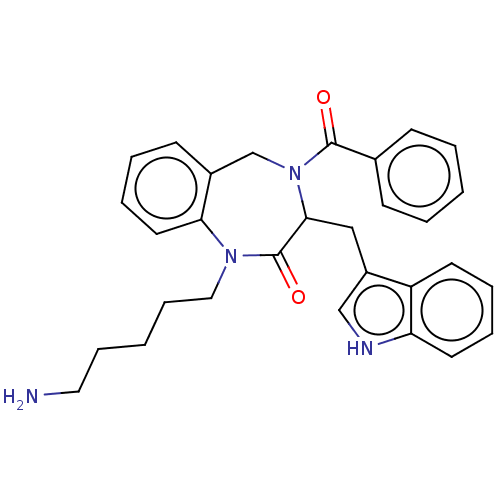
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50214420
(CHEMBL330352)Show SMILES NCCCCCN1c2ccccc2CN(C(Cc2c[nH]c3ccccc23)C1=O)C(=O)c1ccccc1 Show InChI InChI=1S/C30H32N4O2/c31-17-9-2-10-18-33-27-16-8-5-13-23(27)21-34(29(35)22-11-3-1-4-12-22)28(30(33)36)19-24-20-32-26-15-7-6-14-25(24)26/h1,3-8,11-16,20,28,32H,2,9-10,17-19,21,31H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its inhibitory effect against somatostatin receptor in rat cortex membrane using [125I]Tyr3-octreotide as the radioligand |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20G3NBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50214420

(CHEMBL330352)Show SMILES NCCCCCN1c2ccccc2CN(C(Cc2c[nH]c3ccccc23)C1=O)C(=O)c1ccccc1 Show InChI InChI=1S/C30H32N4O2/c31-17-9-2-10-18-33-27-16-8-5-13-23(27)21-34(29(35)22-11-3-1-4-12-22)28(30(33)36)19-24-20-32-26-15-7-6-14-25(24)26/h1,3-8,11-16,20,28,32H,2,9-10,17-19,21,31H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its inhibitory effect against somatostatin receptor in rat cortex membrane using [125I]Tyr3-octreotide as the radioligand |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20G3NBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50214420

(CHEMBL330352)Show SMILES NCCCCCN1c2ccccc2CN(C(Cc2c[nH]c3ccccc23)C1=O)C(=O)c1ccccc1 Show InChI InChI=1S/C30H32N4O2/c31-17-9-2-10-18-33-27-16-8-5-13-23(27)21-34(29(35)22-11-3-1-4-12-22)28(30(33)36)19-24-20-32-26-15-7-6-14-25(24)26/h1,3-8,11-16,20,28,32H,2,9-10,17-19,21,31H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its inhibitory effect against somatostatin receptor in rat cortex membrane using [125I]Tyr3-octreotide as the radioligand |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20G3NBX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020199
(CHEMBL441164 | D-N-Me-Phec(-Cys-Tyr-D-Trp-Orn-Thr-...)Show SMILES CN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC(C)(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O Show InChI InChI=1S/C51H69N11O11S2/c1-27(63)40(43(53)66)60-50(73)42-51(3,4)75-74-26-39(59-45(68)36(54-5)22-29-12-7-6-8-13-29)48(71)57-37(23-30-17-19-32(65)20-18-30)46(69)58-38(24-31-25-55-34-15-10-9-14-33(31)34)47(70)56-35(16-11-21-52)44(67)61-41(28(2)64)49(72)62-42/h6-10,12-15,17-20,25,27-28,35-42,54-55,63-65H,11,16,21-24,26,52H2,1-5H3,(H2,53,66)(H,56,70)(H,57,71)(H,58,69)(H,59,68)(H,60,73)(H,61,67)(H,62,72)/t27-,28-,35+,36-,37+,38+,39+,40+,41+,42-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020212

(CHEMBL405244 | D-Phec(-Cys-Tyr-D-Trp-Lys-Thr-Pen-)...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H](CC(O)=O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C51H67N11O12S2/c1-27(63)41-49(73)62-42(50(74)57-36(43(54)67)24-40(65)66)51(2,3)76-75-26-39(60-44(68)33(53)21-28-11-5-4-6-12-28)48(72)58-37(22-29-16-18-31(64)19-17-29)46(70)59-38(23-30-25-55-34-14-8-7-13-32(30)34)47(71)56-35(45(69)61-41)15-9-10-20-52/h4-8,11-14,16-19,25,27,33,35-39,41-42,55,63-64H,9-10,15,20-24,26,52-53H2,1-3H3,(H2,54,67)(H,56,71)(H,57,74)(H,58,72)(H,59,70)(H,60,68)(H,61,69)(H,62,73)(H,65,66)/t27-,33-,35+,36+,37+,38+,39+,41+,42-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020208

(CHEMBL1795717 | TCTAP)Show SMILES [H][C@](NC(=O)[C@@]1([H])NC(=O)[C@@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@]([H])(CSSC1(C)C)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)([C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C51H69N13O11S2/c1-26(65)39(42(53)68)62-49(75)41-51(3,4)77-76-25-38(61-43(69)33(52)21-28-11-6-5-7-12-28)47(73)59-36(22-29-16-18-31(67)19-17-29)45(71)60-37(23-30-24-57-34-14-9-8-13-32(30)34)46(72)58-35(15-10-20-56-50(54)55)44(70)63-40(27(2)66)48(74)64-41/h5-9,11-14,16-19,24,26-27,33,35-41,57,65-67H,10,15,20-23,25,52H2,1-4H3,(H2,53,68)(H,58,72)(H,59,73)(H,60,71)(H,61,69)(H,62,75)(H,63,70)(H,64,74)(H4,54,55,56)/t26-,27-,33-,35+,36+,37-,38+,39+,40+,41-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020204

(CHEMBL66403 | D-Phec(-Cys-Tyr-Asn-Pen-)Thr-NH2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H46N8O9S2/c1-17(43)26(28(37)46)41-33(51)27-34(2,3)53-52-16-24(40-29(47)21(35)13-18-7-5-4-6-8-18)32(50)38-22(14-19-9-11-20(44)12-10-19)30(48)39-23(15-25(36)45)31(49)42-27/h4-12,17,21-24,26-27,43-44H,13-16,35H2,1-3H3,(H2,36,45)(H2,37,46)(H,38,50)(H,39,48)(H,40,47)(H,41,51)(H,42,49)/t17-,21+,22+,23+,24+,26+,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50213874
(CHEMBL432503)Show SMILES NCCCCCCNC[C@@H]1CN(CCc2c[nH]c3ccccc23)C[C@@H](O)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C37H50N4O3/c38-20-11-1-2-12-21-39-23-32-25-41(22-19-31-24-40-34-18-10-9-17-33(31)34)26-35(42)37(44-28-30-15-7-4-8-16-30)36(32)43-27-29-13-5-3-6-14-29/h3-10,13-18,24,32,35-37,39-40,42H,1-2,11-12,19-23,25-28,38H2/t32-,35-,36-,37-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50213872

(CHEMBL47019)Show SMILES NCCCCCCN[C@H]1CN(CCc2c[nH]c3ccccc23)[C@@H](CO)C(OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C36H48N4O3/c37-20-11-1-2-12-21-38-33-24-40(22-19-30-23-39-32-18-10-9-17-31(30)32)34(25-41)36(43-27-29-15-7-4-8-16-29)35(33)42-26-28-13-5-3-6-14-28/h3-10,13-18,23,33-36,38-39,41H,1-2,11-12,19-22,24-27,37H2/t33-,34-,35+,36?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50213869

(CHEMBL3037859)Show SMILES [H][C@@]1(OCc2ccccc2)C2CN(CCCCCCN)C[C@H](N2CCc2c[nH]c3ccccc23)[C@@]1([H])OCc1ccccc1 |TLB:36:34:22:12.11.20,THB:13:12:22:34.1,2:1:22:12.11.20| Show InChI InChI=1S/C36H46N4O2/c37-20-11-1-2-12-21-39-24-33-35(41-26-28-13-5-3-6-14-28)36(42-27-29-15-7-4-8-16-29)34(25-39)40(33)22-19-30-23-38-32-18-10-9-17-31(30)32/h3-10,13-18,23,33-36,38H,1-2,11-12,19-22,24-27,37H2/t33-,34?,35+,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50213871

(CHEMBL47648)Show SMILES NCCCCCCNC[C@H]1C(OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](O)CN1CCc1c[nH]c2ccccc12 Show InChI InChI=1S/C36H48N4O3/c37-20-11-1-2-12-21-38-24-33-35(42-26-28-13-5-3-6-14-28)36(43-27-29-15-7-4-8-16-29)34(41)25-40(33)22-19-30-23-39-32-18-10-9-17-31(30)32/h3-10,13-18,23,33-36,38-39,41H,1-2,11-12,19-22,24-27,37H2/t33-,34+,35?,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50452485

(CHEMBL2369462)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H]1Cc2ccccc2CN1)C(N)=O Show InChI InChI=1S/C48H62N10O9S2/c1-26(59)39(41(50)61)57-47(67)40-48(2,3)69-68-25-38(56-43(63)35-21-28-10-4-5-11-29(28)23-52-35)46(66)54-36(20-27-15-17-31(60)18-16-27)44(64)55-37(22-30-24-51-33-13-7-6-12-32(30)33)45(65)53-34(42(62)58-40)14-8-9-19-49/h4-7,10-13,15-18,24,26,34-40,51-52,59-60H,8-9,14,19-23,25,49H2,1-3H3,(H2,50,61)(H,53,65)(H,54,66)(H,55,64)(H,56,63)(H,57,67)(H,58,62)/t26-,34+,35-,36-,37+,38+,39+,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50213870

(CHEMBL294905)Show SMILES NCCCCCCNC[C@H]1[C@@H](OCc2ccccc2)C(OCc2ccccc2)[C@H](CO)N1CCc1c[nH]c2ccccc12 Show InChI InChI=1S/C36H48N4O3/c37-20-11-1-2-12-21-38-24-33-35(42-26-28-13-5-3-6-14-28)36(43-27-29-15-7-4-8-16-29)34(25-41)40(33)22-19-30-23-39-32-18-10-9-17-31(30)32/h3-10,13-18,23,33-36,38-39,41H,1-2,11-12,19-22,24-27,37H2/t33-,34-,35+,36?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50213873

(CHEMBL45323)Show SMILES NCCCCCCN1CC2[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)C1CN2CCc1c[nH]c2ccccc12 |TLB:31:30:19.10:7.8| Show InChI InChI=1S/C36H46N4O2/c37-20-11-1-2-12-21-39-24-34-36(42-27-29-15-7-4-8-16-29)35(41-26-28-13-5-3-6-14-28)33(39)25-40(34)22-19-30-23-38-32-18-10-9-17-31(30)32/h3-10,13-18,23,33-36,38H,1-2,11-12,19-22,24-27,37H2/t33?,34?,35-,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its potency to displace 3-[125I]- - Tyr11-SRIF-14 from somatostatin receptor on membranes of rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22B9166 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020207

(CHEMBL2369473 | Gly-D-Ticc(-Cys-Tyr-D-Trp-Orn-Thr-...)Show SMILES [H][C@]1(NC(=O)[C@H](CCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H]1Cc2ccccc2CN1C(=O)CN)[C@@H](C)O Show InChI InChI=1S/C53H70N12O12S2/c1-27(66)42(45(56)70)62-52(77)44-53(3,4)79-78-26-39(61-50(75)40-22-30-10-5-6-11-31(30)25-65(40)41(69)23-55)49(74)59-37(20-29-15-17-33(68)18-16-29)47(72)60-38(21-32-24-57-35-13-8-7-12-34(32)35)48(73)58-36(14-9-19-54)46(71)63-43(28(2)67)51(76)64-44/h5-8,10-13,15-18,24,27-28,36-40,42-44,57,66-68H,9,14,19-23,25-26,54-55H2,1-4H3,(H2,56,70)(H,58,73)(H,59,74)(H,60,72)(H,61,75)(H,62,77)(H,63,71)(H,64,76)/t27-,28-,36+,37-,38+,39+,40-,42+,43+,44-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020197

(CHEMBL2369475 | TCTOP)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@@H]1Cc2ccccc2CN1)[C@@H](C)O Show InChI InChI=1S/C46H69N11O11S2/c1-24(58)35(38(49)61)55-45(68)37-46(3,4)70-69-23-34(54-41(64)32-21-27-10-5-6-11-28(27)22-50-32)43(66)53-33(20-26-14-16-29(60)17-15-26)42(65)52-31(13-9-19-48)39(62)51-30(12-7-8-18-47)40(63)56-36(25(2)59)44(67)57-37/h5-6,10-11,14-17,24-25,30-37,50,58-60H,7-9,12-13,18-23,47-48H2,1-4H3,(H2,49,61)(H,51,62)(H,52,65)(H,53,66)(H,54,64)(H,55,68)(H,56,63)(H,57,67)/t24-,25-,30+,31+,32+,33+,34+,35+,36+,37-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50212468
(CHEMBL28415)Show SMILES NCCCCCOCC1OC(OCCc2c[nH]c3ccccc23)C(OCc2ccccc2)C1OCc1ccccc1 Show InChI InChI=1S/C34H42N2O5/c35-19-10-3-11-20-37-25-31-32(39-23-26-12-4-1-5-13-26)33(40-24-27-14-6-2-7-15-27)34(41-31)38-21-18-28-22-36-30-17-9-8-16-29(28)30/h1-2,4-9,12-17,22,31-34,36H,3,10-11,18-21,23-25,35H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Radioligand binding studies on rat cortex membranes using 125 I-Tyrs-octreotide as ligand was determined |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X350M0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020203
(CHEMBL2369474 | D-Ticc(-Cys-Tyr-D-Trp-Lys-Pen-)Thr...)Show SMILES [H][C@]1(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@@H]1Cc2ccccc2CN1)[C@@H](C)O |wU:66.69,16.16,30.32,57.62,5.5,wD:49.56,56.59,42.66,76.83,1.0,(-3.25,5.4,;-1.52,4.68,;-2.9,3.98,;-3.98,2.9,;-4.98,3.62,;-4.69,1.52,;-6.16,2.01,;-6.47,3.51,;-7.94,3.99,;-8.26,5.5,;-9.72,5.98,;-9.98,7.18,;-10.64,5.15,;-4.93,,;-4.69,-1.52,;-5.86,-1.9,;-3.98,-2.89,;-5.24,-3.8,;-6.65,-3.17,;-6.95,-1.67,;-8.45,-1.51,;-9.09,-2.91,;-10.57,-3.36,;-10.9,-4.88,;-9.77,-5.92,;-8.27,-5.46,;-7.96,-3.95,;-2.9,-3.98,;-1.52,-4.68,;-1.9,-5.85,;,-4.92,;-0,-6.47,;-1.34,-7.24,;-1.35,-8.78,;-2.68,-9.55,;-4.01,-8.77,;-5.08,-9.39,;-4.01,-7.23,;-2.67,-6.47,;1.52,-4.68,;2.89,-3.98,;3.62,-4.98,;3.98,-2.89,;4.68,-1.52,;4.92,,;4.68,1.52,;3.98,2.9,;3.78,4.11,;5.08,2.33,;2.89,3.98,;1.52,4.68,;,4.92,;,6.15,;3.8,5.24,;5.03,5.11,;3.17,6.65,;4.08,7.9,;5.61,7.73,;6.33,8.73,;6.11,6.61,;3.45,9.3,;2.22,9.43,;4.17,10.3,;5.23,-3.81,;5.07,-5.34,;3.95,-5.84,;6.32,-6.25,;6.16,-7.79,;7.4,-8.7,;7.24,-10.23,;8.49,-11.13,;9.89,-10.51,;10.05,-8.98,;8.81,-8.08,;8.97,-6.54,;7.72,-5.62,;-2,6.16,;-1.18,7.07,;-3.21,6.41,)| Show InChI InChI=1S/C52H69N13O11S2/c1-26(66)40(43(53)69)63-50(76)42-52(3,4)78-77-25-39(62-45(71)36-21-29-10-5-6-11-30(29)23-58-36)48(74)60-37(20-28-15-17-32(68)18-16-28)46(72)61-38(22-31-24-57-34-13-8-7-12-33(31)34)47(73)59-35(14-9-19-56-51(54)55)44(70)64-41(27(2)67)49(75)65-42/h5-8,10-13,15-18,24,26-27,35-42,57-58,66-68H,9,14,19-23,25H2,1-4H3,(H2,53,69)(H,59,73)(H,60,74)(H,61,72)(H,62,71)(H,63,76)(H,64,70)(H,65,75)(H4,54,55,56)/t26-,27-,35+,36+,37+,38+,39+,40+,41+,42-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
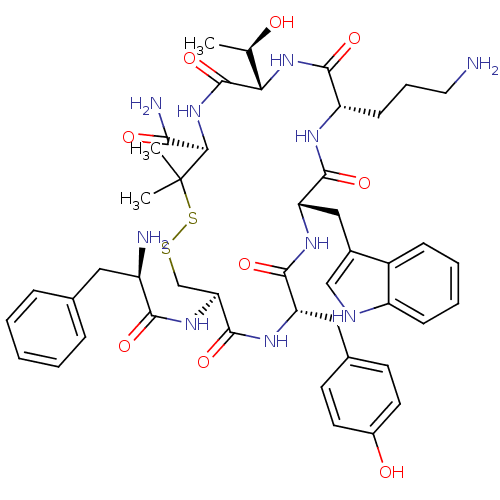
(RAT) | BDBM50020215
(CHEMBL541457 | D-Phec(-Cys-Tyr-D-Trp-Orn-The-Pen-)...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC(C)(C)[C@H](NC1=O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C46H60N10O9S2/c1-25(57)37-45(65)56-38(39(49)59)46(2,3)67-66-24-36(54-40(60)31(48)20-26-10-5-4-6-11-26)44(64)52-34(21-27-15-17-29(58)18-16-27)42(62)53-35(22-28-23-50-32-13-8-7-12-30(28)32)43(63)51-33(14-9-19-47)41(61)55-37/h4-8,10-13,15-18,23,25,31,33-38,50,57-58H,9,14,19-22,24,47-48H2,1-3H3,(H2,49,59)(H,51,63)(H,52,64)(H,53,62)(H,54,60)(H,55,61)(H,56,65)/t25-,31-,33+,34+,35+,36+,37+,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50020206

(CHEMBL266829 | CTOP)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C45H69N11O11S2/c1-24(57)34(37(49)60)54-44(67)36-45(3,4)69-68-23-33(53-38(61)29(48)21-26-11-6-5-7-12-26)42(65)52-32(22-27-15-17-28(59)18-16-27)41(64)51-31(14-10-20-47)39(62)50-30(13-8-9-19-46)40(63)55-35(25(2)58)43(66)56-36/h5-7,11-12,15-18,24-25,29-36,57-59H,8-10,13-14,19-23,46-48H2,1-4H3,(H2,49,60)(H,50,62)(H,51,64)(H,52,65)(H,53,61)(H,54,67)(H,55,63)(H,56,66)/t24-,25-,29+,30+,31+,32+,33+,34+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity in competition with [125I]- CGP-23996 in somatostatin receptor binding to rat brain membrane |
J Med Chem 31: 2170-7 (1988)
BindingDB Entry DOI: 10.7270/Q2J38RJX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































