

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445384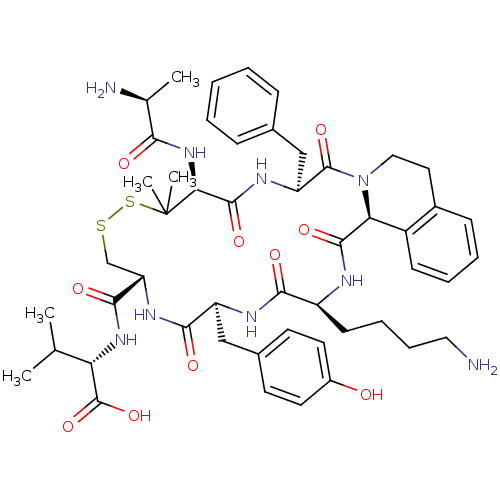 (CHEMBL3104469) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445385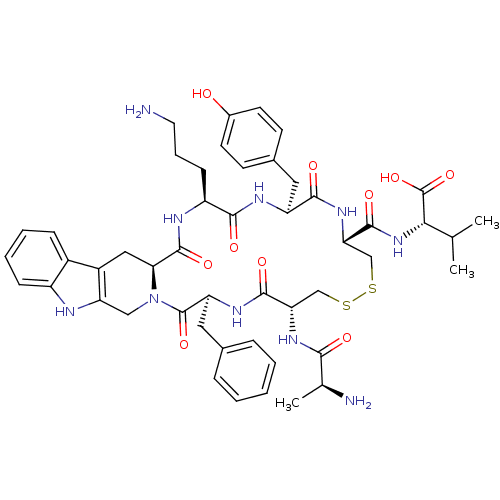 (CHEMBL3104468) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445368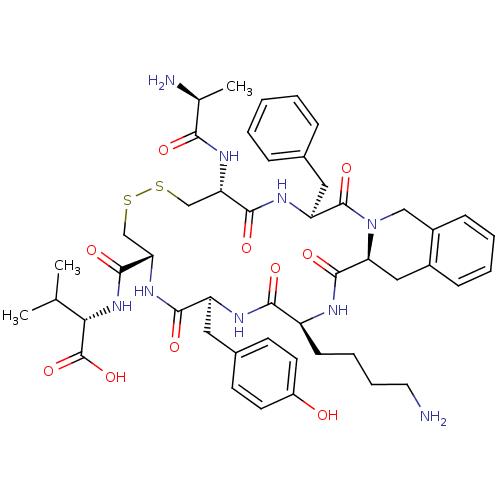 (CHEMBL3104466) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445378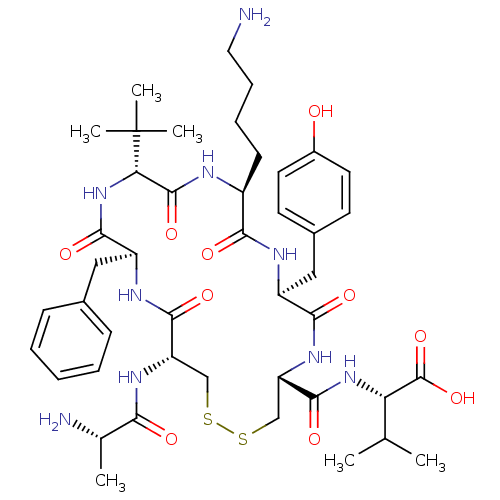 (CHEMBL3104642) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445386 (CHEMBL3104641) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445384 (CHEMBL3104469) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445385 (CHEMBL3104468) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445368 (CHEMBL3104466) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445378 (CHEMBL3104642) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445386 (CHEMBL3104641) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50558696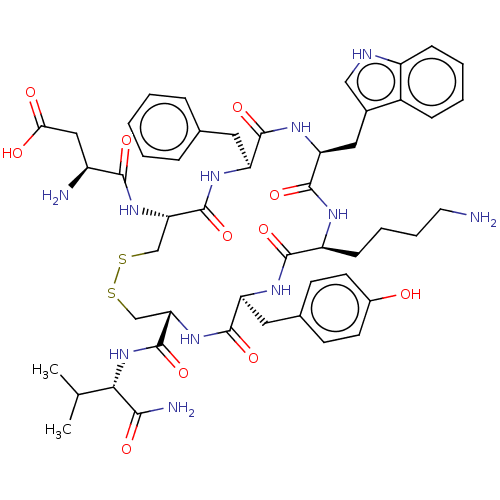 (CHEMBL4777970) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I-Tyr9]-U-II from human U-IIR expressed in HEK293-A cells incubated for 30 mins by gamma counting based competition radioligand b... | Citation and Details BindingDB Entry DOI: 10.7270/Q2377DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302270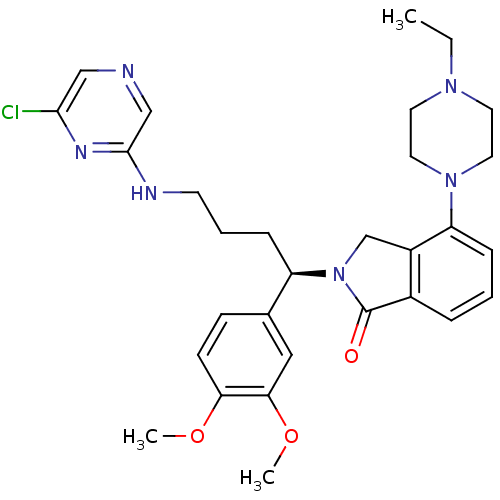 ((R)-2-(4-(6-chloropyrazin-2-ylamino)-1-(3,4-dimeth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302257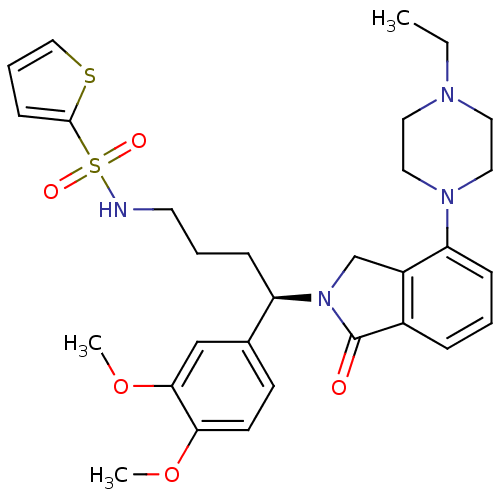 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302264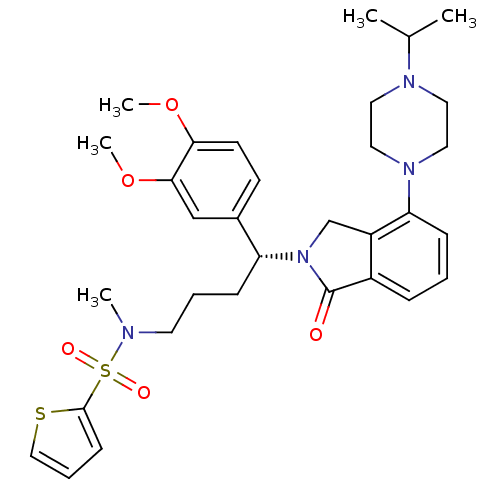 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50558695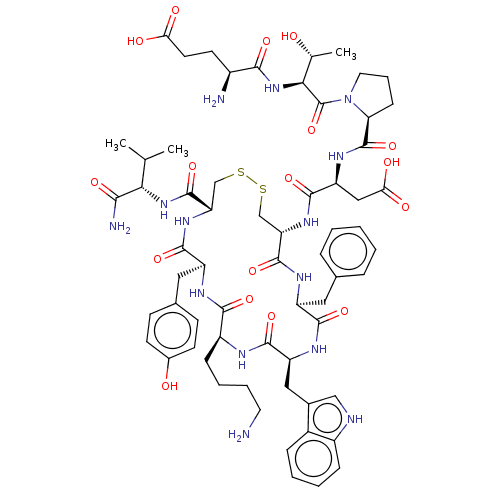 (CHEMBL4741230) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I-Tyr9]-U-II from human U-IIR expressed in HEK293-A cells incubated for 30 mins by gamma counting based competition radioligand b... | Citation and Details BindingDB Entry DOI: 10.7270/Q2377DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543457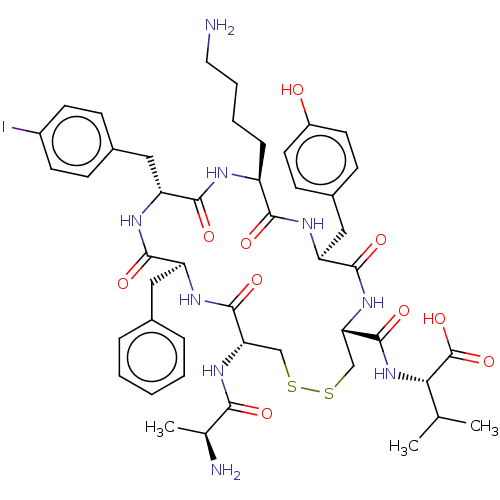 (CHEMBL4635039) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302258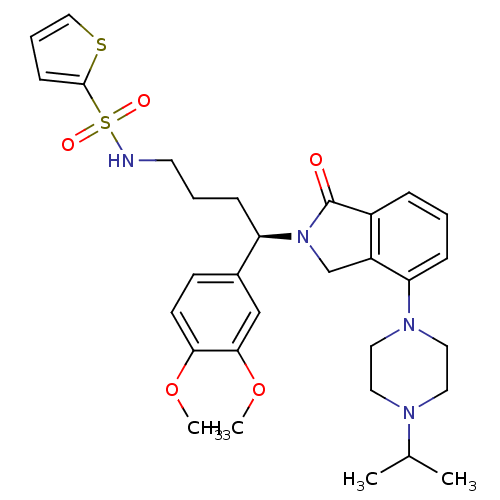 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543458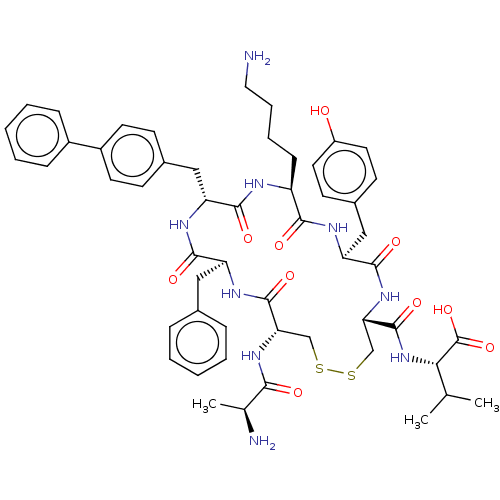 (CHEMBL4642307) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302250 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302272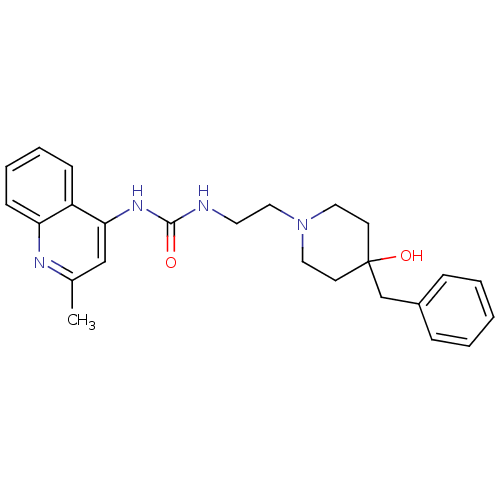 (1-(2-(4-benzyl-4-hydroxypiperidin-1-yl)ethyl)-3-(2...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human urotensin 2 receptor | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459265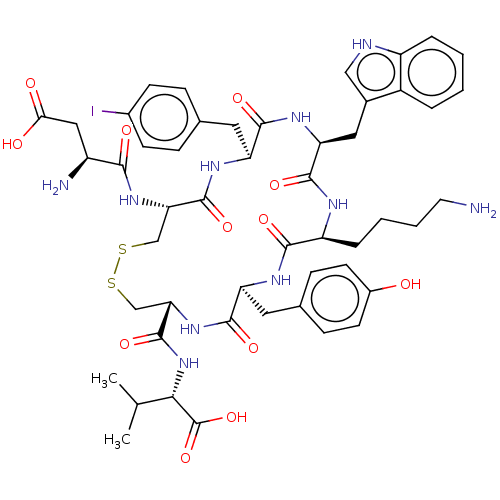 (CHEMBL4205274) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302266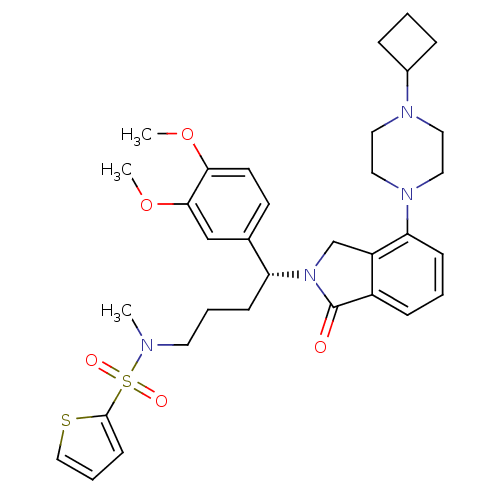 ((R)-N-(4-(4-(4-cyclobutylpiperazin-1-yl)-1-oxoisoi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302271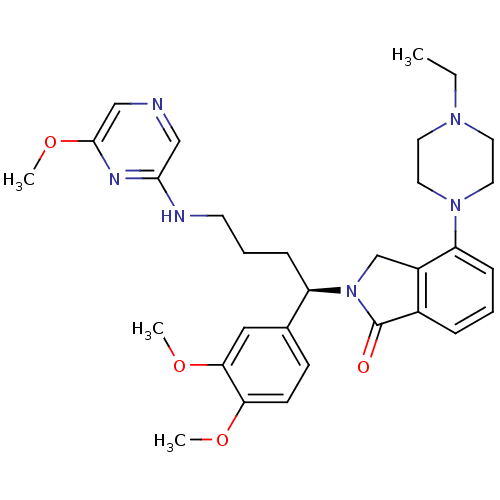 ((R)-2-(1-(3,4-dimethoxyphenyl)-4-(6-methoxypyrazin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50252659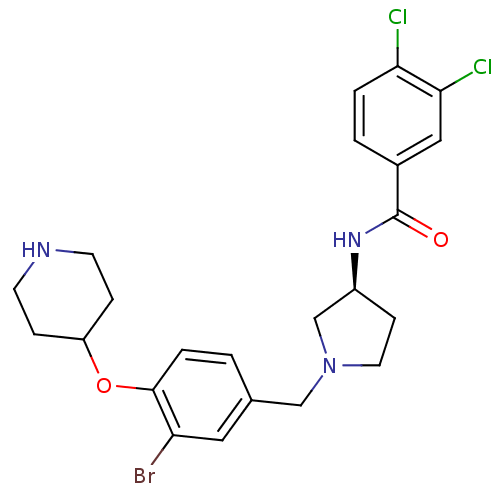 ((S)-N-(1-(3-bromo-4-(piperidin-4-yloxy)benzyl)pyrr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... | Bioorg Med Chem Lett 18: 3950-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.019 BindingDB Entry DOI: 10.7270/Q2FT8KTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302249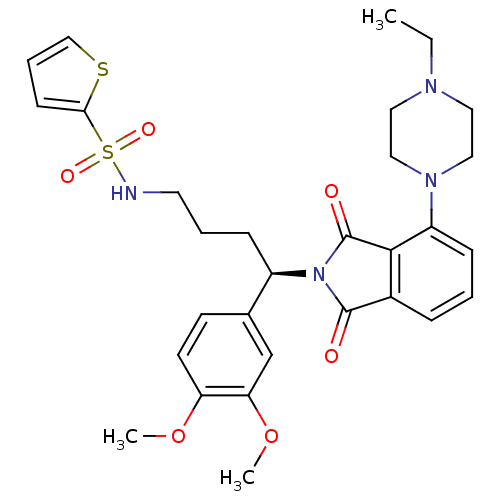 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445383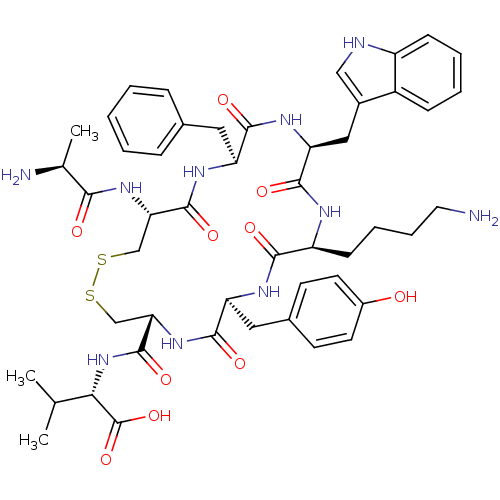 (CHEMBL3104471) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459261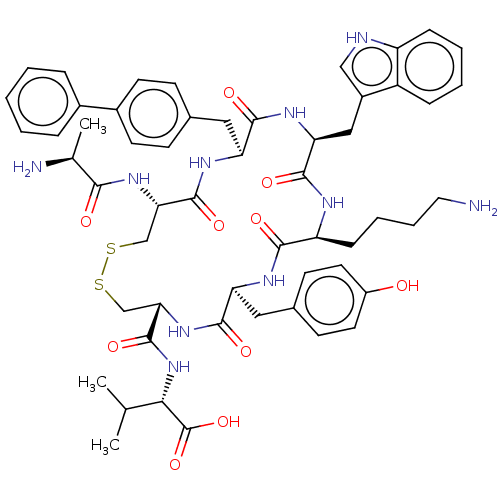 (CHEMBL4208184) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459266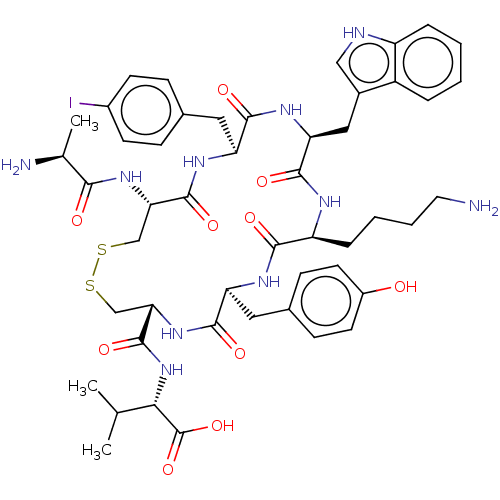 (CHEMBL4217514) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302268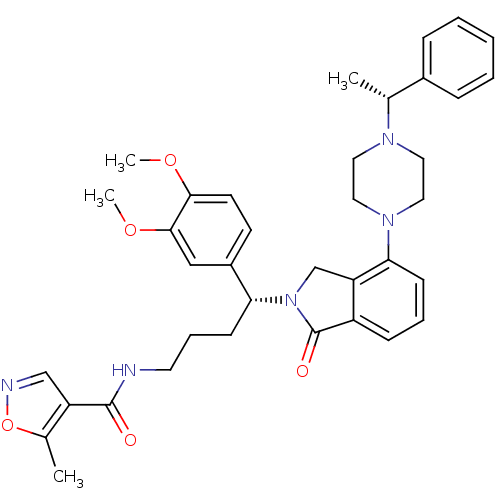 (CHEMBL578206 | N-((R)-4-(3,4-dimethoxyphenyl)-4-(1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302260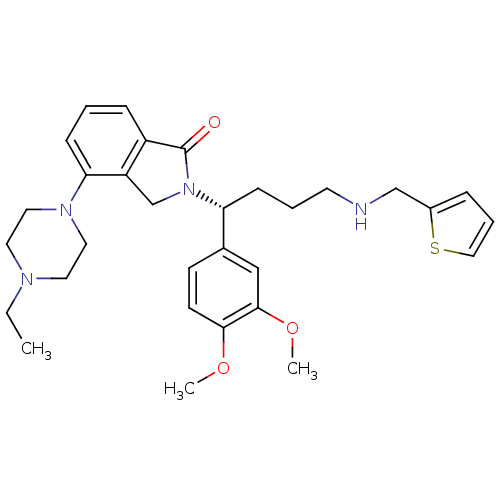 ((R)-2-(1-(3,4-dimethoxyphenyl)-4-(thiophen-2-ylmet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50508365 (CHEMBL4457118) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Displacement of Eu-labelled UT2 from recombinant human UT2 receptor expressed in HEK293 cell membranes preincubated for 10 mins followed by Eu-labell... | Bioorg Med Chem Lett 29: 577-580 (2019) Article DOI: 10.1016/j.bmcl.2018.12.058 BindingDB Entry DOI: 10.7270/Q2445QS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878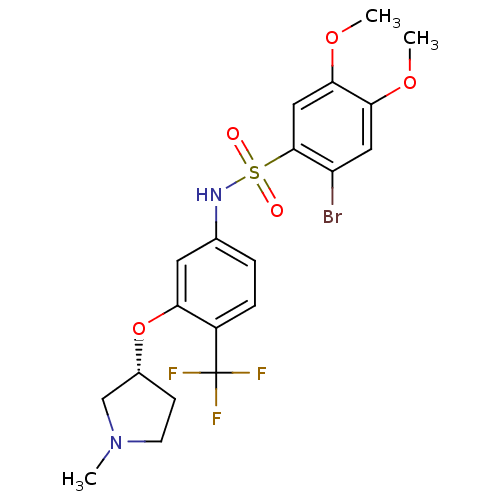 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-U2 from human recombinant urotensin2 receptor expressed in human Chem-2 cells after 4 hrs by scintillation proximity assay | Bioorg Med Chem Lett 23: 2177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.01.105 BindingDB Entry DOI: 10.7270/Q2XS5WSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302259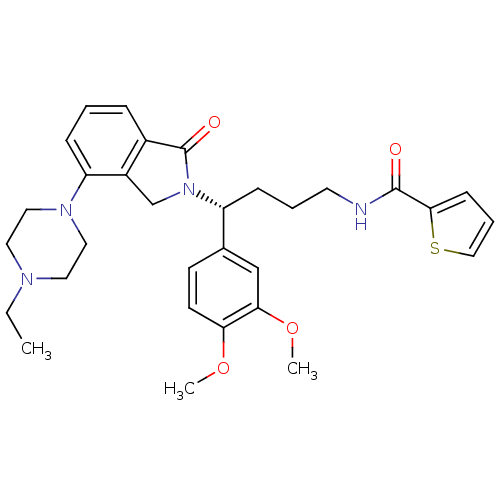 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302261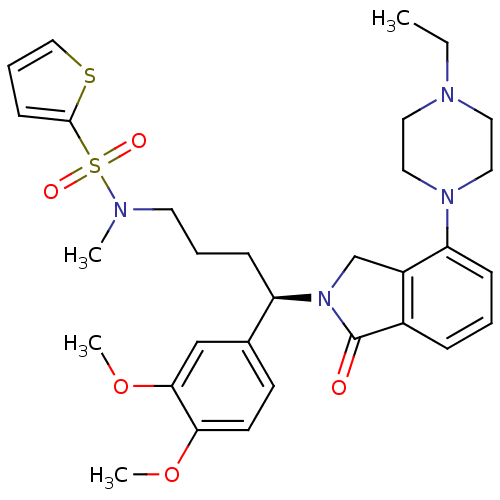 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543461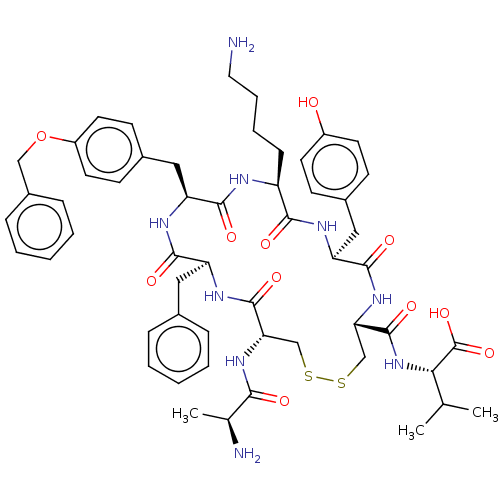 (CHEMBL4638346) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302257 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at urotensin 2 receptor in human RMS13 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobilization aft... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at urotensin 2 receptor in human RMS13 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobilization aft... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445376 (CHEMBL3104644) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445376 (CHEMBL3104644) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50508375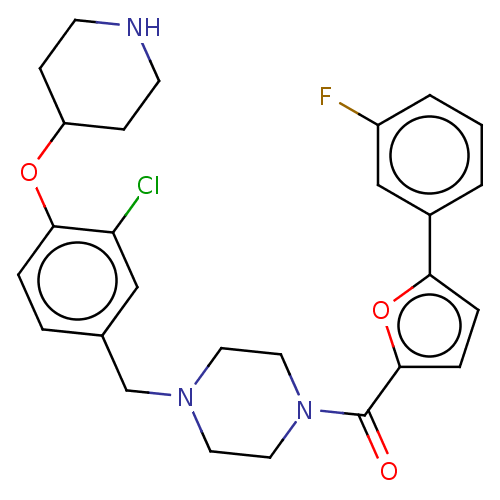 (CHEMBL4525919) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Displacement of Eu-labelled UT2 from recombinant human UT2 receptor expressed in HEK293 cell membranes preincubated for 10 mins followed by Eu-labell... | Bioorg Med Chem Lett 29: 577-580 (2019) Article DOI: 10.1016/j.bmcl.2018.12.058 BindingDB Entry DOI: 10.7270/Q2445QS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50224715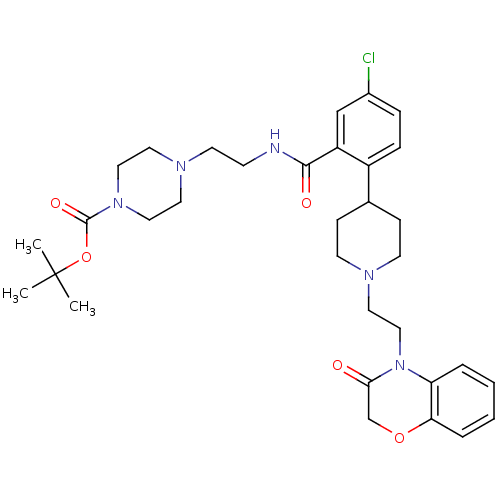 (CHEMBL396443 | tert-butyl 4-(2-(5-chloro-2-(1-(2-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Antagonist activity at rat UT receptor transfected in CHOK1 cells assessed as calcium mobilization by FLIPR method | Bioorg Med Chem Lett 17: 6489-92 (2007) Article DOI: 10.1016/j.bmcl.2007.09.092 BindingDB Entry DOI: 10.7270/Q2C53KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445370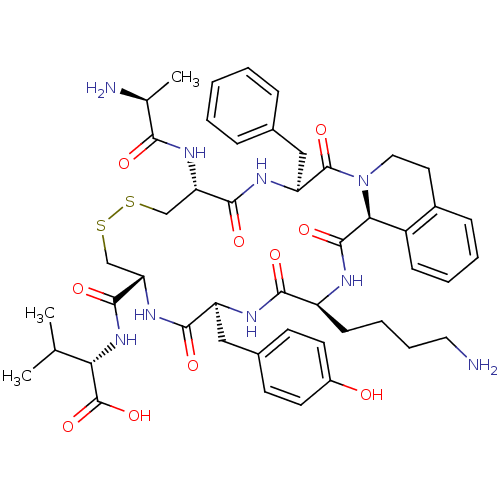 (CHEMBL3104464) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445370 (CHEMBL3104464) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302252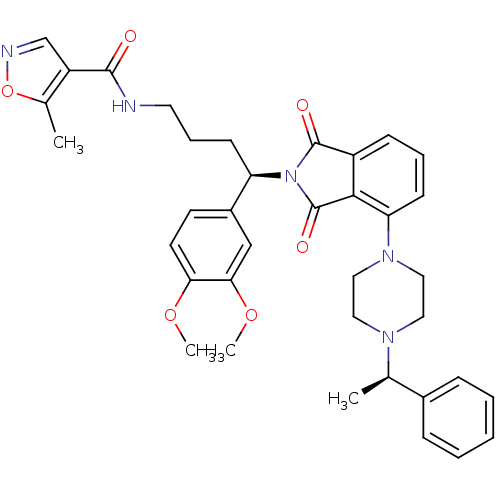 (CHEMBL568764 | N-((R)-4-(3,4-dimethoxyphenyl)-4-(1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302254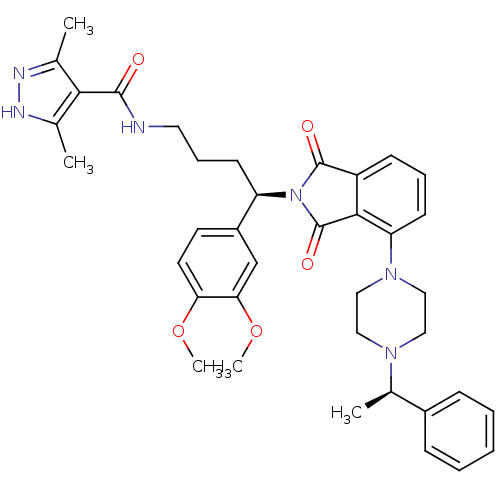 (CHEMBL565388 | N-((R)-4-(3,4-dimethoxyphenyl)-4-(1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302253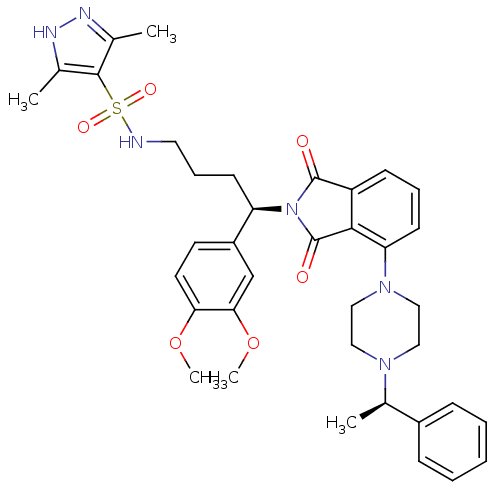 (CHEMBL567759 | N-((R)-4-(3,4-dimethoxyphenyl)-4-(1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543459 (CHEMBL4643905) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445383 (CHEMBL3104471) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50378580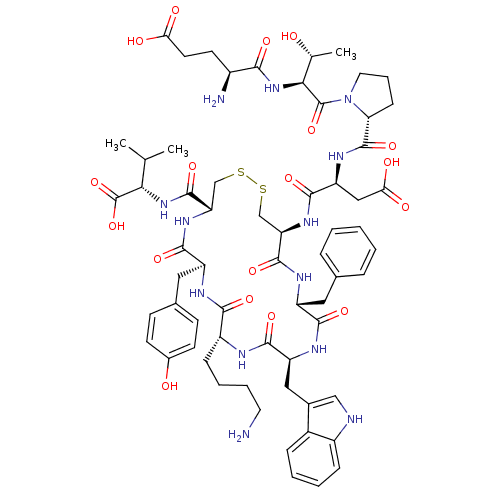 (CHEMBL437430) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysis | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50036148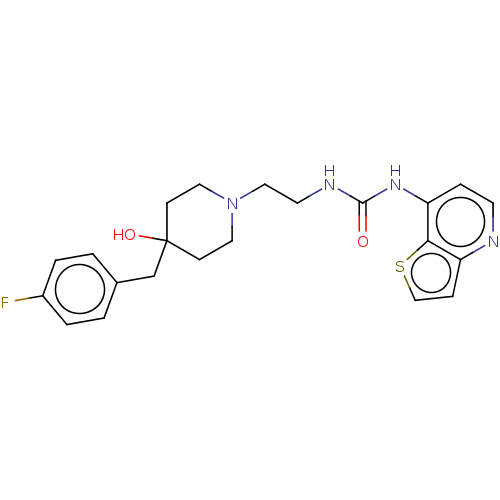 (CHEMBL3358687) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Displacement of europium-labeled urotensin-II from human urotensin-2 receptor expressed in HEK293 cells by TRF assay | Bioorg Med Chem Lett 24: 5832-5 (2014) Article DOI: 10.1016/j.bmcl.2014.09.089 BindingDB Entry DOI: 10.7270/Q2H133M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 342 total ) | Next | Last >> |