

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (RAT) | BDBM50445368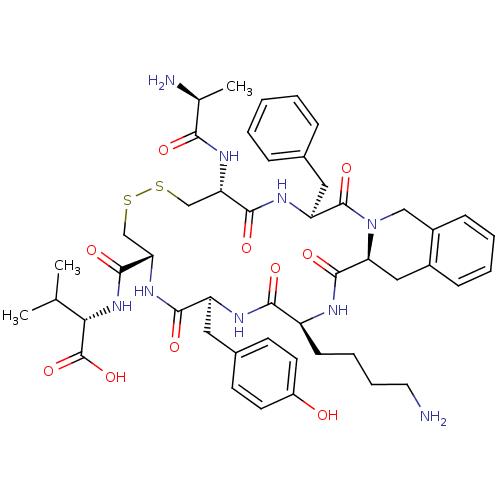 (CHEMBL3104466) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445375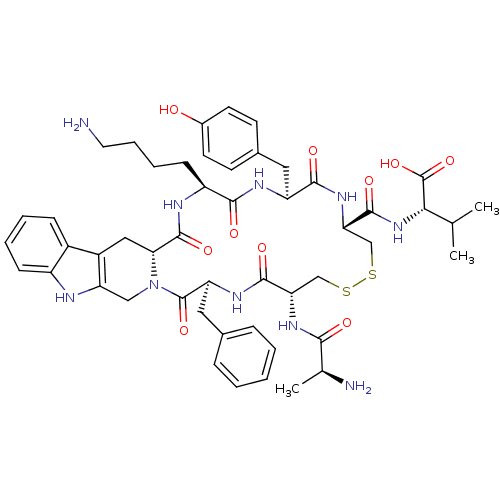 (CHEMBL3104459) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445377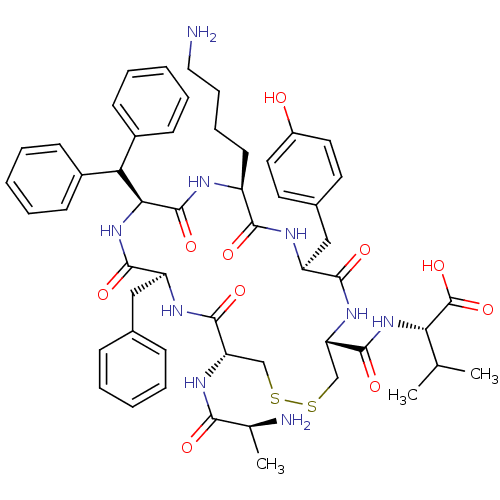 (CHEMBL3104643) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445378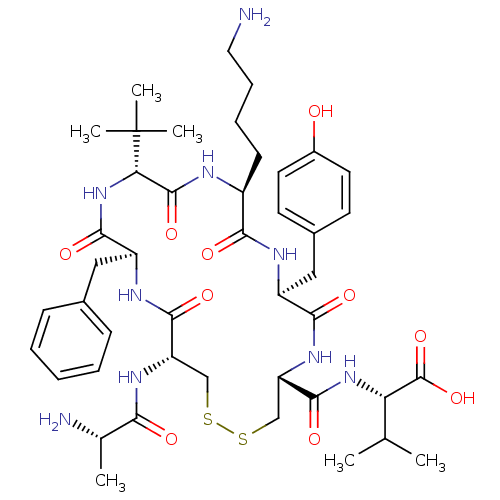 (CHEMBL3104642) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445379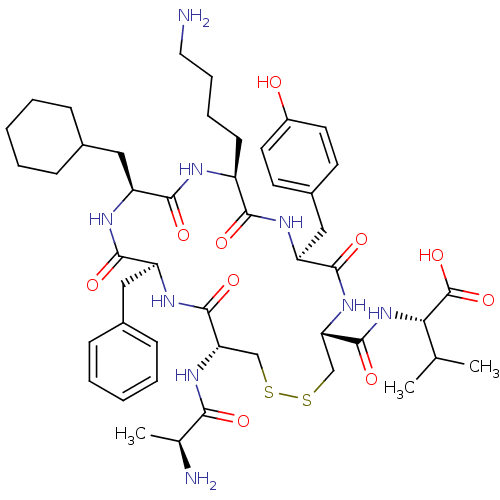 (CHEMBL3104640) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445368 (CHEMBL3104466) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445375 (CHEMBL3104459) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445377 (CHEMBL3104643) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445378 (CHEMBL3104642) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50445379 (CHEMBL3104640) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048701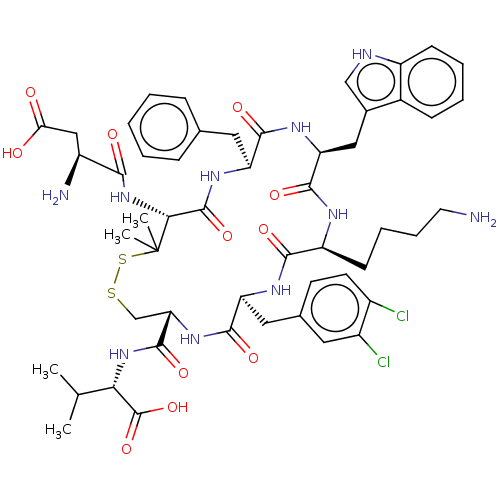 (CHEMBL3315148) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0126 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048697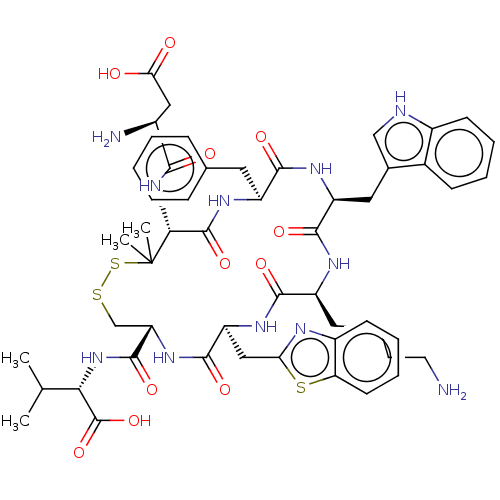 (CHEMBL3315144) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0195 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320463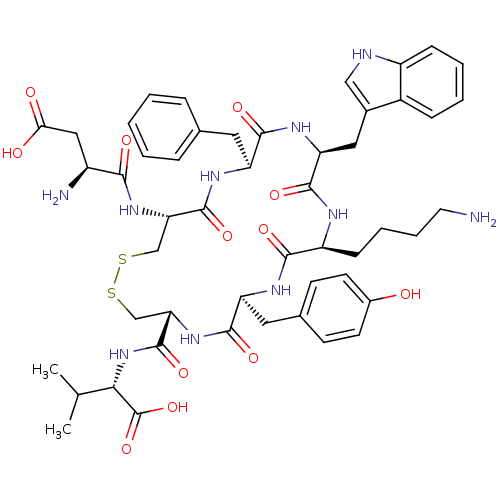 (CHEMBL218994 | D[CFWKYC]V | H-Asp-Cys-Phe-Trp-Lys-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50089666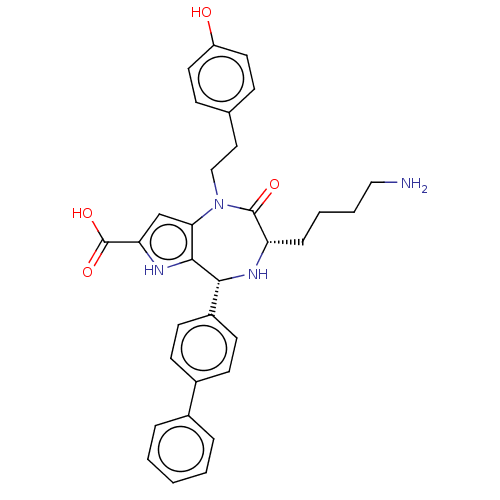 (CHEMBL3577310) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as human urotensin-2 EC50 for induction of aortic ring contrac... | J Med Chem 58: 4624-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00162 BindingDB Entry DOI: 10.7270/Q2CR5W3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320456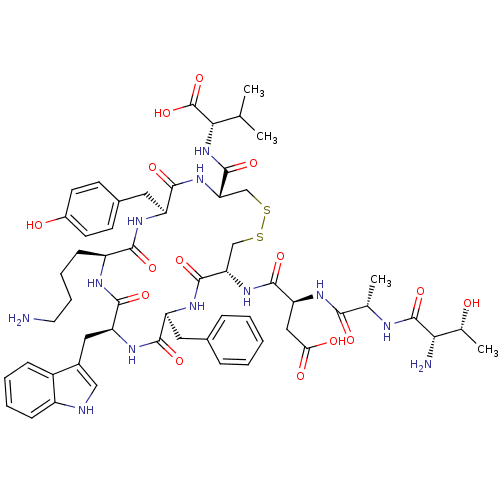 (CHEMBL1165734 | TAD[CFWKYC]V) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320457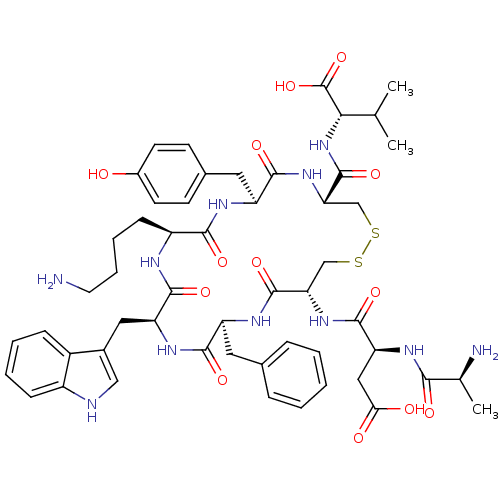 (AD[CFWKYC]V | CHEMBL1163460) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320454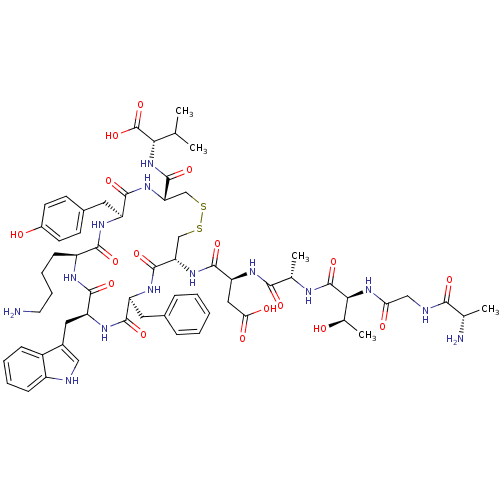 (AGTAD[CFWKYC]V | CHEMBL1163463) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50413761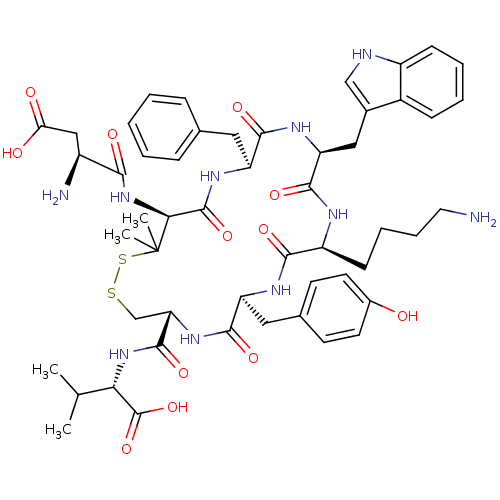 (CHEMBL390094) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Agonist activity at UT2 receptor in Albino rat aorta assessed as induction of aortic contraction | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320455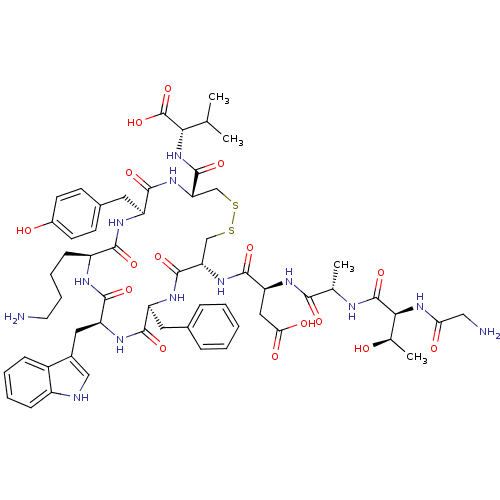 (CHEMBL1163467 | GTAD[CFWKYC]V) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320472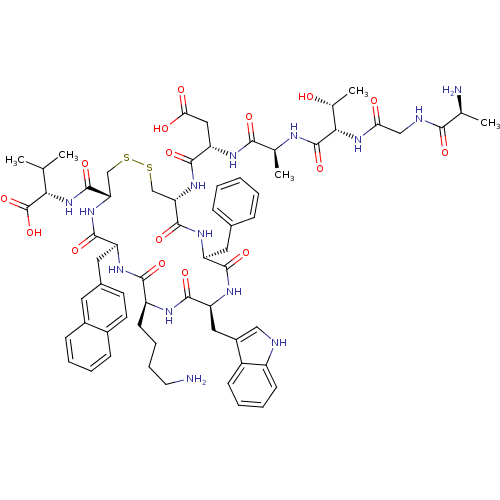 ((3S,6S,9S,15S)-3-((4R,7S,10S,13S,16S,19R)-13-((1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048691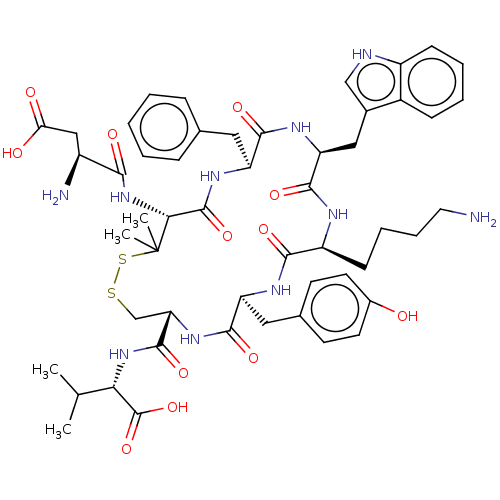 (CHEMBL3315139) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320462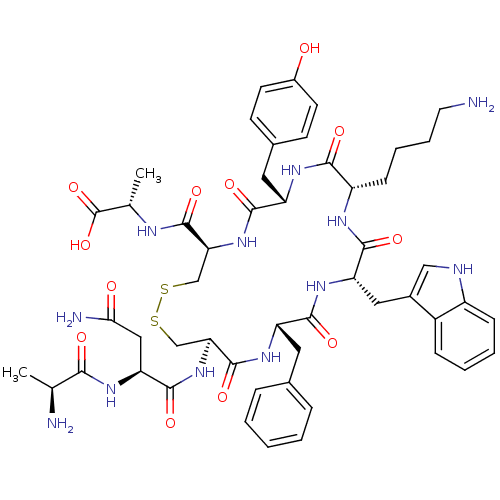 (AD[CFWKYC]A | CHEMBL1165767) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273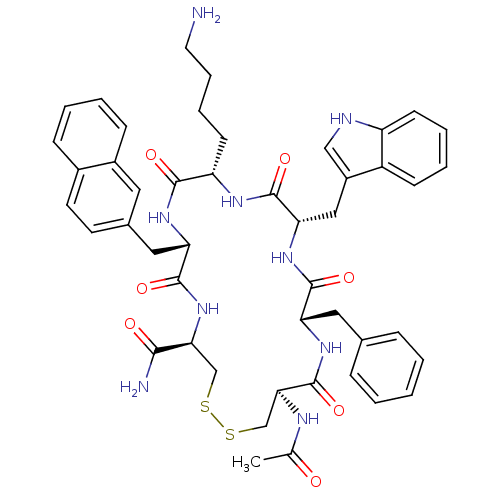 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza... | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320458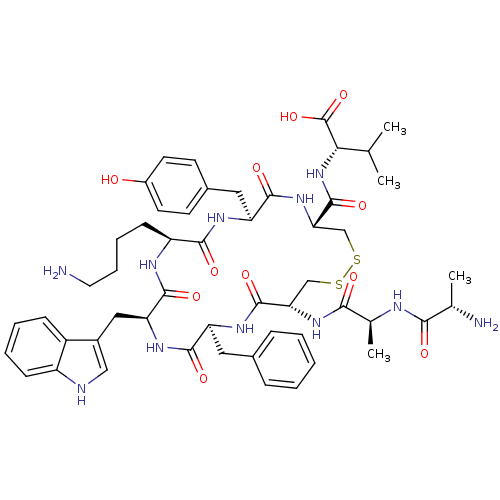 (AA[CFWKYC]V | CHEMBL1165735) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048705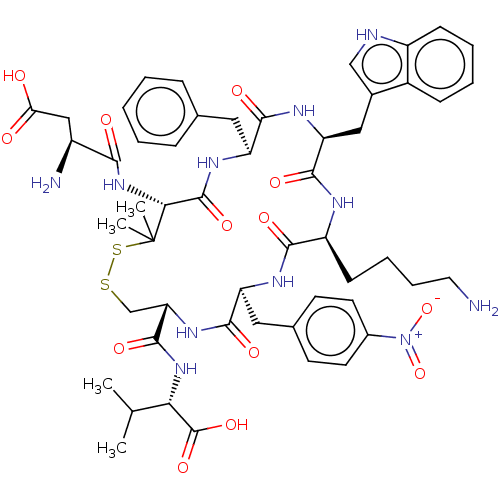 (CHEMBL3315152) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50413764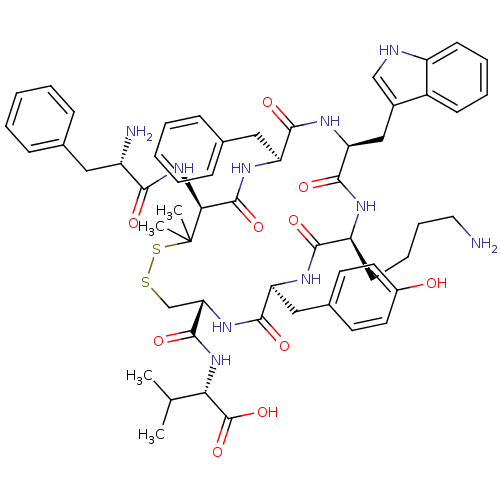 (CHEMBL504097) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.661 | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Agonist activity at UT2 receptor in Albino rat aorta assessed as induction of aortic contraction | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048692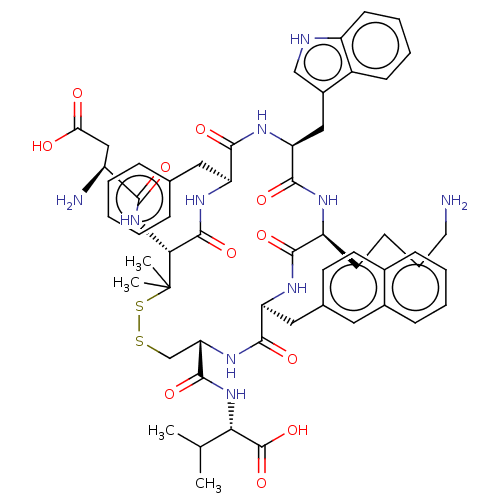 (CHEMBL3315140) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.724 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320464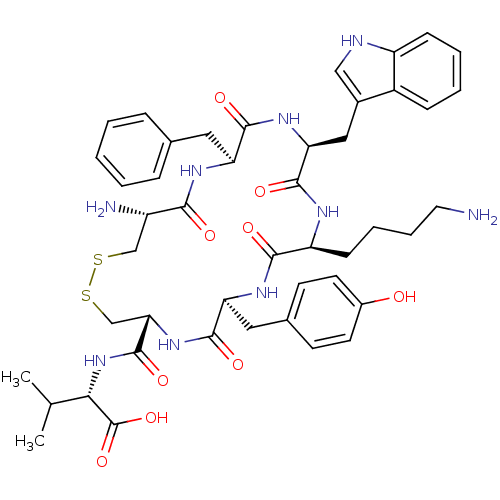 (CHEMBL1163473 | [CFWKYC]V) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048699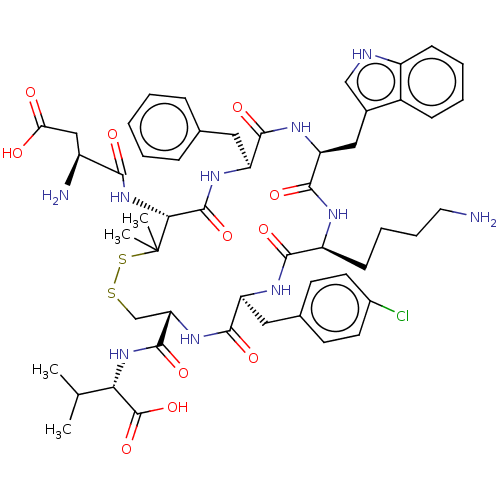 (CHEMBL3315146) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.813 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50378580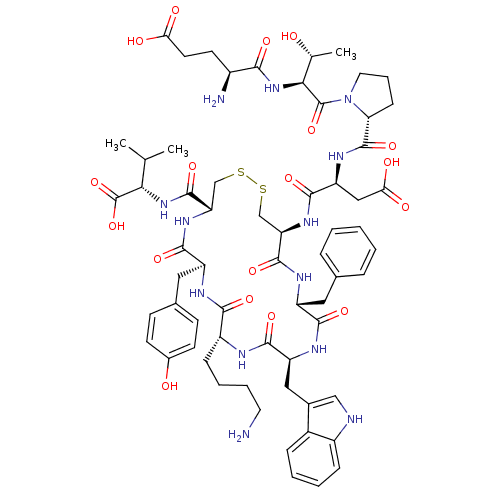 (CHEMBL437430) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.832 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50378580 (CHEMBL437430) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.832 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstriction | J Med Chem 56: 9612-22 (2014) Article DOI: 10.1021/jm401153j BindingDB Entry DOI: 10.7270/Q2TD9ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048703 (CHEMBL3315150) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50459262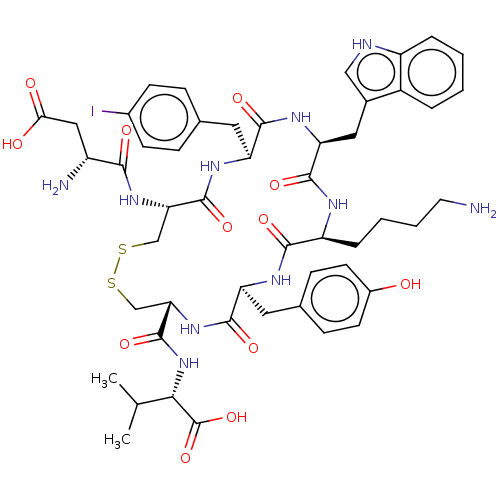 (CHEMBL4213538) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at UT receptor in Sprague-Dawley rat thoracic aortic ring assessed as reduction in urotensin-2-induced contraction by measuring p... | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50459264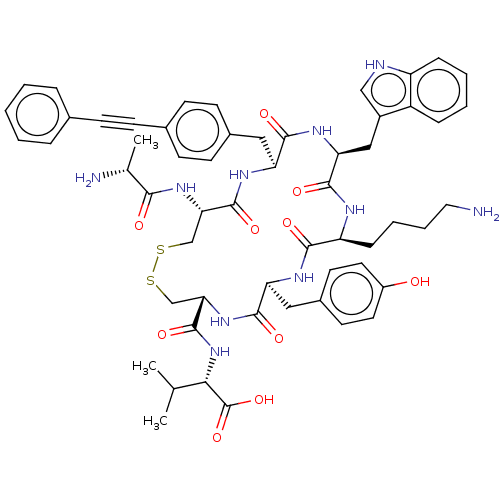 (CHEMBL4216988) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at UT receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50413777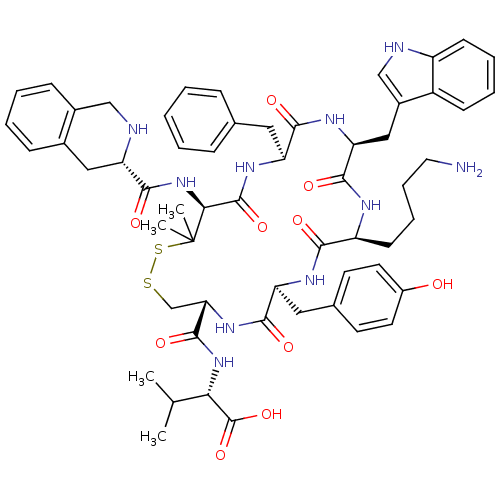 (CHEMBL524855) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Agonist activity at UT2 receptor in Albino rat aorta assessed as induction of aortic contraction | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50413766 (CHEMBL510618) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Agonist activity at UT2 receptor in Albino rat aorta assessed as induction of aortic contraction | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269957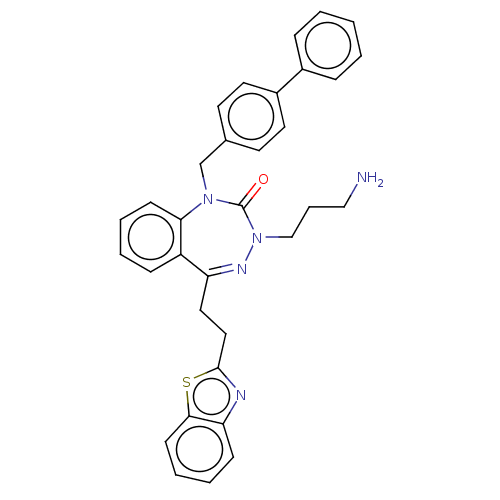 (CHEMBL4068478) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50089660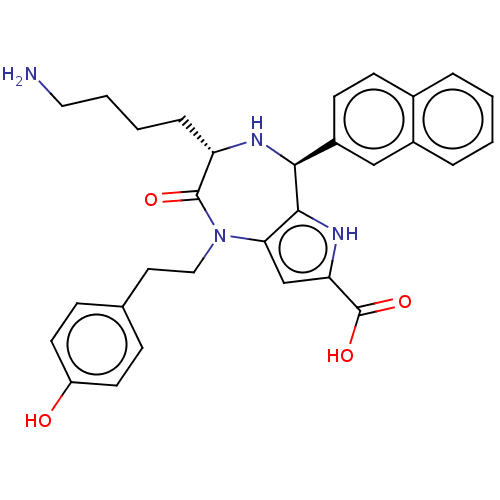 (CHEMBL3577311) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as human urotensin-2 EC50 for induction of aortic ring contrac... | J Med Chem 58: 4624-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00162 BindingDB Entry DOI: 10.7270/Q2CR5W3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50089660 (CHEMBL3577311) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as human urotensin-2 pEC50 for induction of aortic ring contra... | J Med Chem 58: 4624-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00162 BindingDB Entry DOI: 10.7270/Q2CR5W3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320471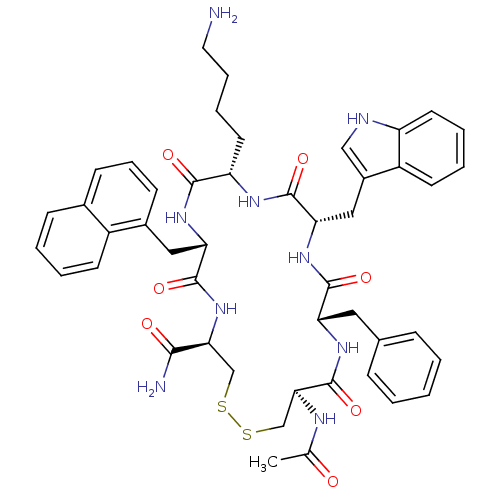 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50185139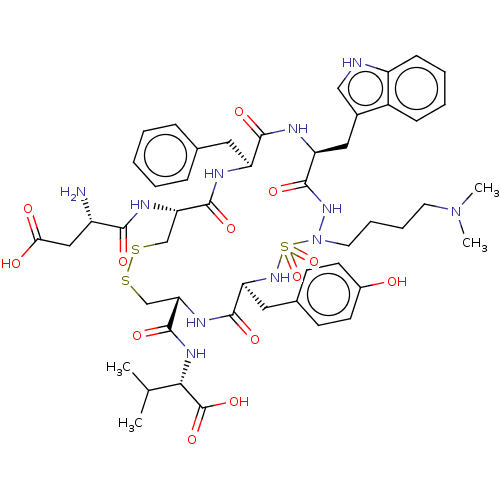 (CHEMBL3823939) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Antagonist activity at UT2 receptor in Sprague-Dawley rat thoracic aorta ring assessed as pEC50 for hU2-induced aortic contractions at 10'-5M relativ... | J Med Chem 59: 4740-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00108 BindingDB Entry DOI: 10.7270/Q2SB47Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320466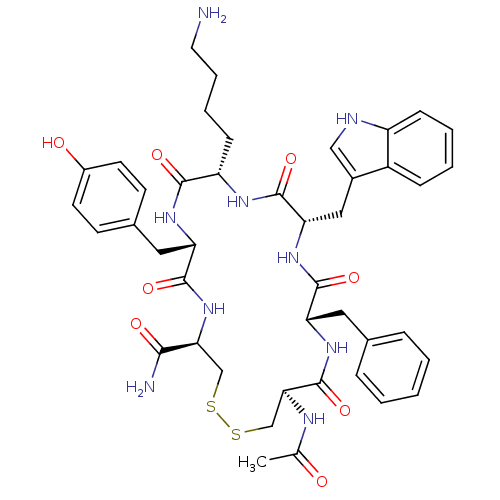 (Ac-[CFWKYC]-NH2 | CHEMBL1165794) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320465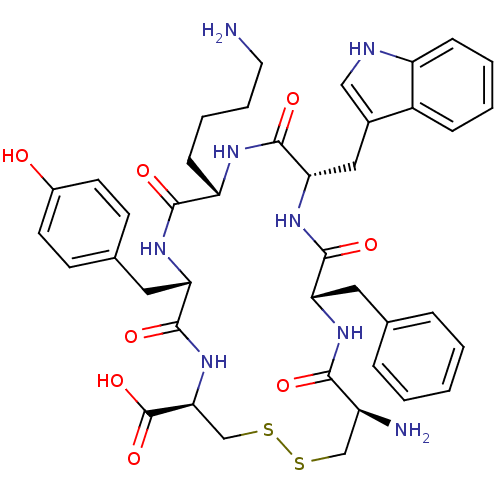 (CFWKYC | CHEMBL1165793) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50459267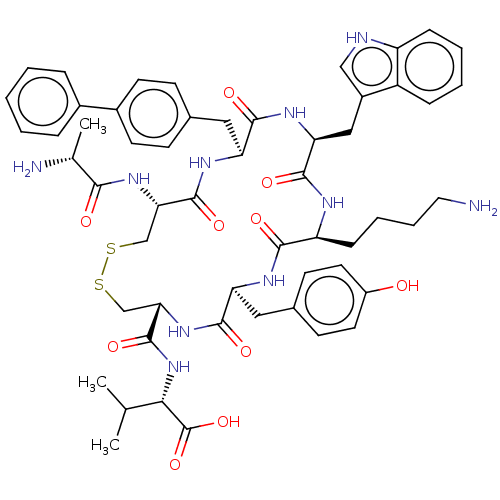 (CHEMBL4209654) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Agonist activity at UT receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320469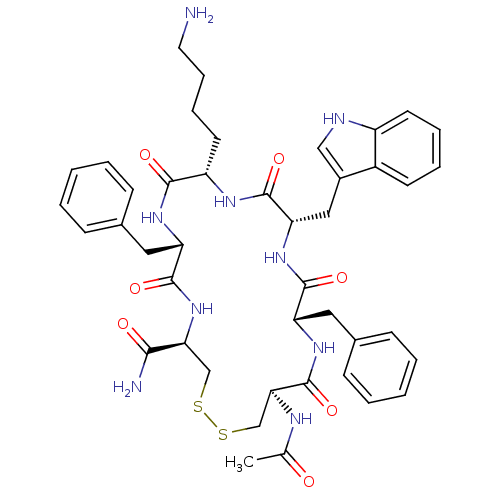 (Ac-[CFWKFC]-NH2 | CHEMBL1163471) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50413760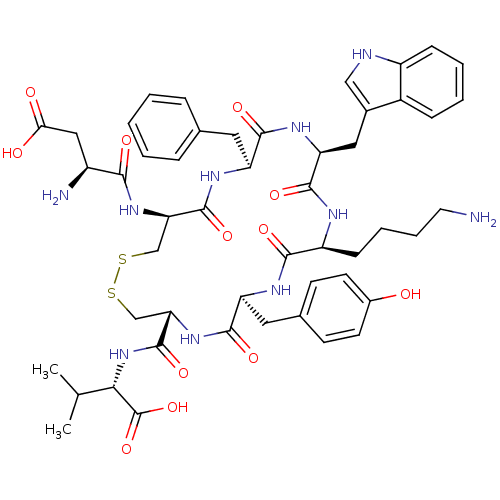 (CHEMBL426020) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Agonist activity at UT2 receptor in Albino rat aorta assessed as induction of aortic contraction | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50089670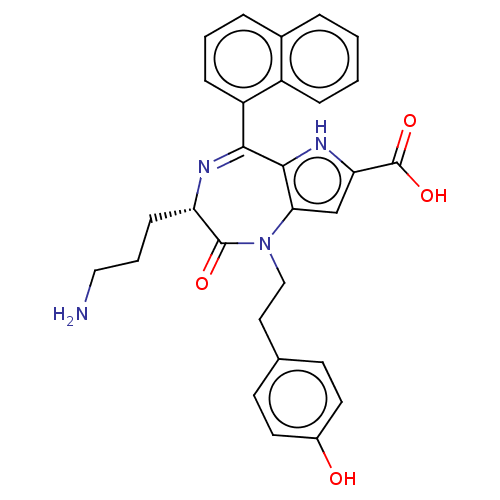 (CHEMBL3577301) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as human urotensin-2 pEC50 for induction of aortic ring contra... | J Med Chem 58: 4624-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00162 BindingDB Entry DOI: 10.7270/Q2CR5W3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50185137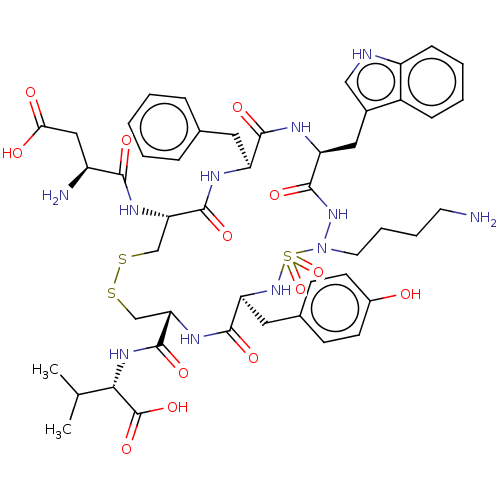 (CHEMBL3823569) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Antagonist activity at UT2 receptor in Sprague-Dawley rat thoracic aorta ring assessed as pEC50 for hU2-induced aortic contractions at 10'-5M relativ... | J Med Chem 59: 4740-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00108 BindingDB Entry DOI: 10.7270/Q2SB47Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50048700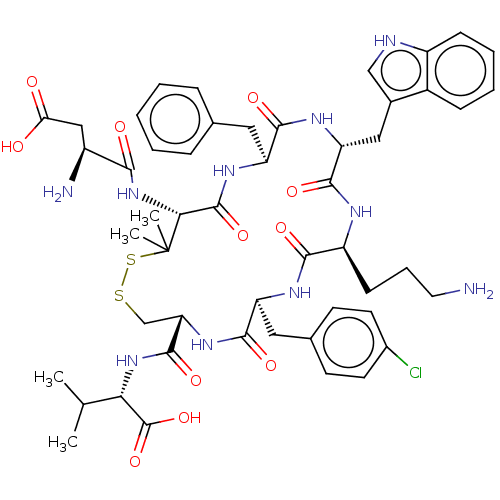 (CHEMBL3315147) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Agonist activity at UT receptor in rat aorta assessed as contraction | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 243 total ) | Next | Last >> |