 Found 167 hits of ki data for polymerid = 4970
Found 167 hits of ki data for polymerid = 4970 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219586
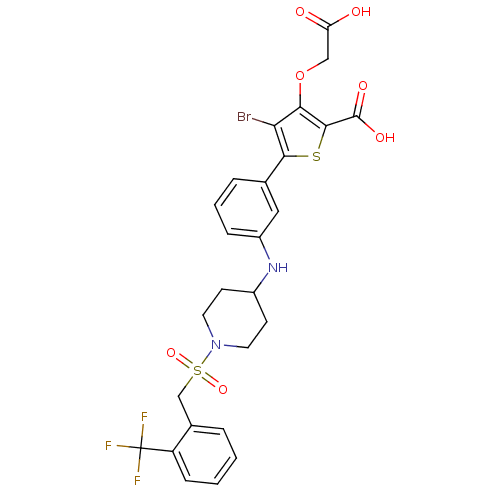
(4-bromo-3-(carboxymethoxy)-5-{3-[(1-{[2-(trifluoro...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2C(F)(F)F)c1 Show InChI InChI=1S/C26H24BrF3N2O7S2/c27-21-22(39-13-20(33)34)24(25(35)36)40-23(21)15-5-3-6-18(12-15)31-17-8-10-32(11-9-17)41(37,38)14-16-4-1-2-7-19(16)26(28,29)30/h1-7,12,17,31H,8-11,13-14H2,(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219577
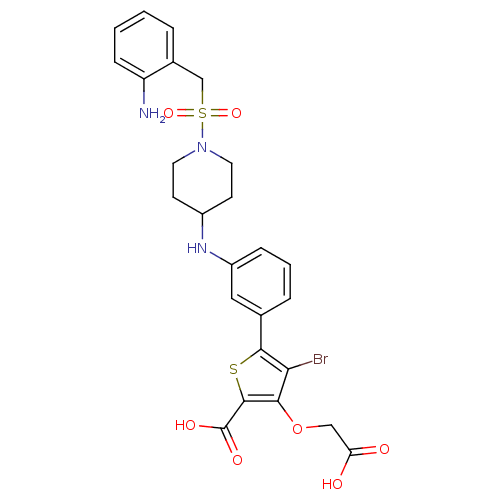
(5-[3-({1-[(2-aminobenzyl)sulfonyl]piperidin-4-yl}a...)Show SMILES Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C25H26BrN3O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)15-5-3-6-18(12-15)28-17-8-10-29(11-9-17)38(34,35)14-16-4-1-2-7-19(16)27/h1-7,12,17,28H,8-11,13-14,27H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219584
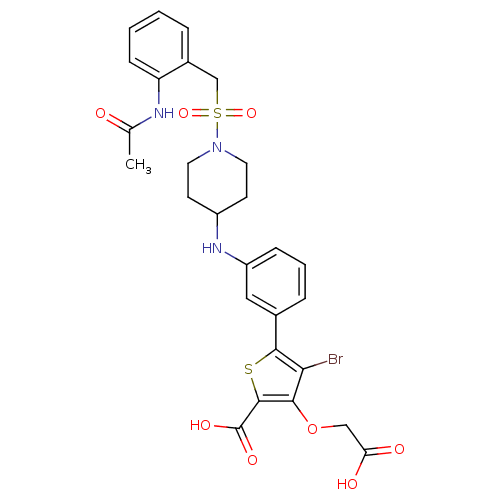
(5-{3-[(1-{[2-(acetylamino)benzyl]sulfonyl}piperidi...)Show SMILES CC(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C27H28BrN3O8S2/c1-16(32)29-21-8-3-2-5-18(21)15-41(37,38)31-11-9-19(10-12-31)30-20-7-4-6-17(13-20)25-23(28)24(39-14-22(33)34)26(40-25)27(35)36/h2-8,13,19,30H,9-12,14-15H2,1H3,(H,29,32)(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219588
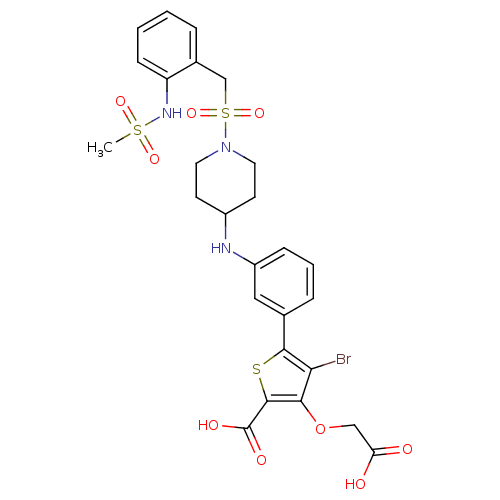
(4-bromo-3-(carboxymethoxy)-5-(3-{[1-({2-[(methylsu...)Show SMILES CS(=O)(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C26H28BrN3O9S3/c1-41(35,36)29-20-8-3-2-5-17(20)15-42(37,38)30-11-9-18(10-12-30)28-19-7-4-6-16(13-19)24-22(27)23(39-14-21(31)32)25(40-24)26(33)34/h2-8,13,18,28-29H,9-12,14-15H2,1H3,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219575
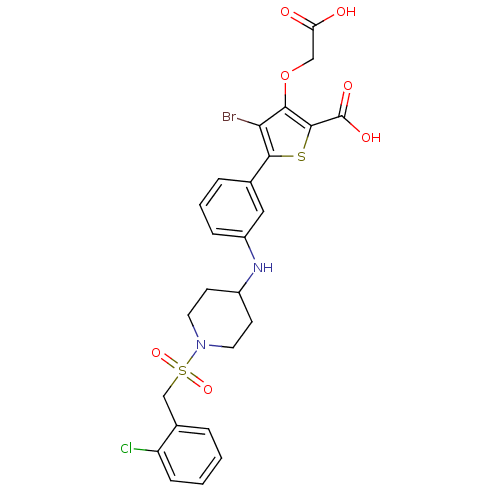
(3-carboxymethoxy-5-{3-[1-(2-chlorobenzenesulfonyl)...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2Cl)c1 Show InChI InChI=1S/C25H24BrClN2O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)15-5-3-6-18(12-15)28-17-8-10-29(11-9-17)38(34,35)14-16-4-1-2-7-19(16)27/h1-7,12,17,28H,8-11,13-14H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM199180
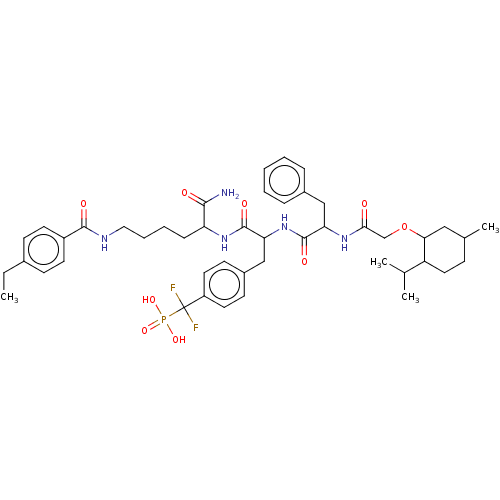
(US9217012, 10)Show SMILES CCc1ccc(cc1)C(=O)NCCCCC(NC(=O)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)COC1CC(C)CCC1C(C)C)C(N)=O Show InChI InChI=1S/C46H62F2N5O9P/c1-5-31-15-19-34(20-16-31)43(56)50-24-10-9-13-37(42(49)55)52-45(58)39(27-33-17-21-35(22-18-33)46(47,48)63(59,60)61)53-44(57)38(26-32-11-7-6-8-12-32)51-41(54)28-62-40-25-30(4)14-23-36(40)29(2)3/h6-8,11-12,15-22,29-30,36-40H,5,9-10,13-14,23-28H2,1-4H3,(H2,49,55)(H,50,56)(H,51,54)(H,52,58)(H,53,57)(H2,59,60,61) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.10 | -11.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation
US Patent
| Assay Description
PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... |
US Patent US9217012 (2015)
BindingDB Entry DOI: 10.7270/Q2FX788H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM199180

(US9217012, 10)Show SMILES CCc1ccc(cc1)C(=O)NCCCCC(NC(=O)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)COC1CC(C)CCC1C(C)C)C(N)=O Show InChI InChI=1S/C46H62F2N5O9P/c1-5-31-15-19-34(20-16-31)43(56)50-24-10-9-13-37(42(49)55)52-45(58)39(27-33-17-21-35(22-18-33)46(47,48)63(59,60)61)53-44(57)38(26-32-11-7-6-8-12-32)51-41(54)28-62-40-25-30(4)14-23-36(40)29(2)3/h6-8,11-12,15-22,29-30,36-40H,5,9-10,13-14,23-28H2,1-4H3,(H2,49,55)(H,50,56)(H,51,54)(H,52,58)(H,53,57)(H2,59,60,61) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.30 | -11.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation
US Patent
| Assay Description
PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... |
US Patent US9217012 (2015)
BindingDB Entry DOI: 10.7270/Q2FX788H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219566
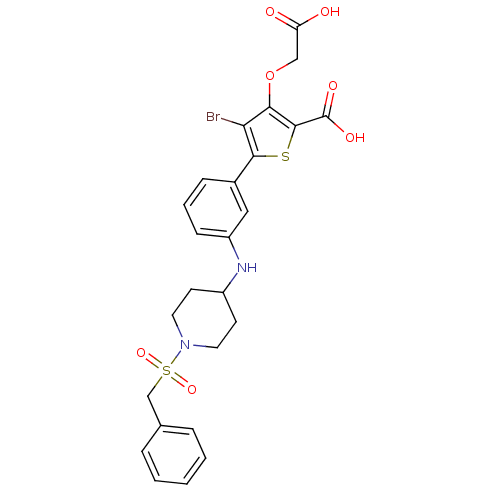
(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219570
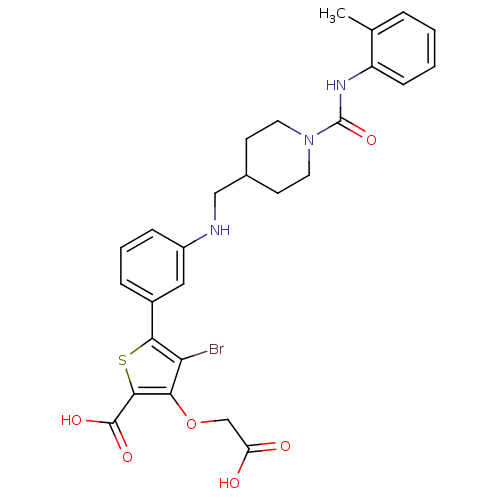
(4-bromo-3-carboxymethoxy-5-{3-[(1-o-tolylcarbamoyl...)Show SMILES Cc1ccccc1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C27H28BrN3O6S/c1-16-5-2-3-8-20(16)30-27(36)31-11-9-17(10-12-31)14-29-19-7-4-6-18(13-19)24-22(28)23(37-15-21(32)33)25(38-24)26(34)35/h2-8,13,17,29H,9-12,14-15H2,1H3,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219566

(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219585
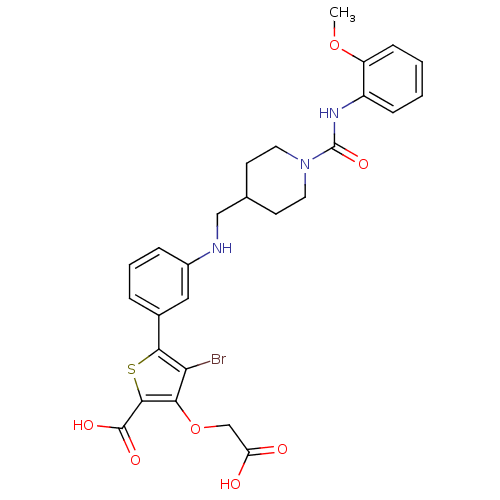
(4-bromo-3-carboxymethoxy-5-(3-{[1-(2-methoxyphenyl...)Show SMILES COc1ccccc1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C27H28BrN3O7S/c1-37-20-8-3-2-7-19(20)30-27(36)31-11-9-16(10-12-31)14-29-18-6-4-5-17(13-18)24-22(28)23(38-15-21(32)33)25(39-24)26(34)35/h2-8,13,16,29H,9-12,14-15H2,1H3,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219567
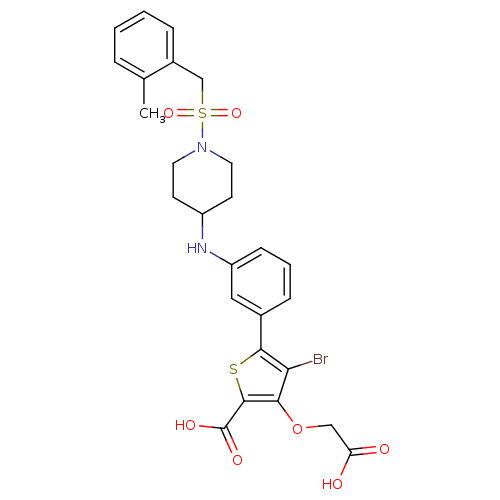
(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2-methylbenz...)Show SMILES Cc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C26H27BrN2O7S2/c1-16-5-2-3-6-18(16)15-38(34,35)29-11-9-19(10-12-29)28-20-8-4-7-17(13-20)24-22(27)23(36-14-21(30)31)25(37-24)26(32)33/h2-8,13,19,28H,9-12,14-15H2,1H3,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219568

(4-bromo-3-carboxymethoxy-5-(3-{[1-(2-chlorophenylc...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NCC2CCN(CC2)C(=O)Nc2ccccc2Cl)c1 Show InChI InChI=1S/C26H25BrClN3O6S/c27-21-22(37-14-20(32)33)24(25(34)35)38-23(21)16-4-3-5-17(12-16)29-13-15-8-10-31(11-9-15)26(36)30-19-7-2-1-6-18(19)28/h1-7,12,15,29H,8-11,13-14H2,(H,30,36)(H,32,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219565
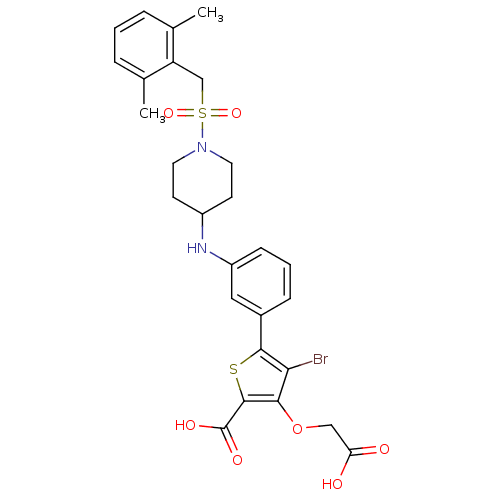
(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2,6-dimethyl...)Show SMILES Cc1cccc(C)c1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C27H29BrN2O7S2/c1-16-5-3-6-17(2)21(16)15-39(35,36)30-11-9-19(10-12-30)29-20-8-4-7-18(13-20)25-23(28)24(37-14-22(31)32)26(38-25)27(33)34/h3-8,13,19,29H,9-12,14-15H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219576
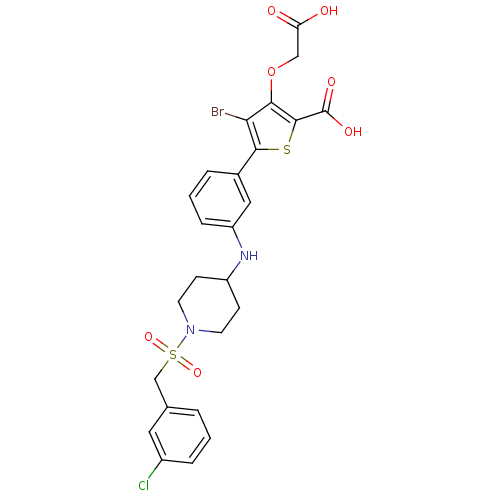
(4-bromo-3-(carboxymethoxy)-5-(3-(1-(3-chlorobenzyl...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2cccc(Cl)c2)c1 Show InChI InChI=1S/C25H24BrClN2O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)16-4-2-6-19(12-16)28-18-7-9-29(10-8-18)38(34,35)14-15-3-1-5-17(27)11-15/h1-6,11-12,18,28H,7-10,13-14H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219569
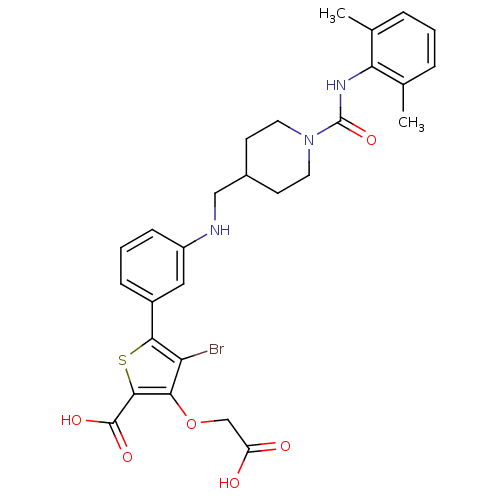
(4-bromo-3-carboxymethoxy-5-(3-{[1-(2,6-dimethylphe...)Show SMILES Cc1cccc(C)c1NC(=O)N1CCC(CNc2cccc(c2)-c2sc(C(O)=O)c(OCC(O)=O)c2Br)CC1 Show InChI InChI=1S/C28H30BrN3O6S/c1-16-5-3-6-17(2)23(16)31-28(37)32-11-9-18(10-12-32)14-30-20-8-4-7-19(13-20)25-22(29)24(38-15-21(33)34)26(39-25)27(35)36/h3-8,13,18,30H,9-12,14-15H2,1-2H3,(H,31,37)(H,33,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219580
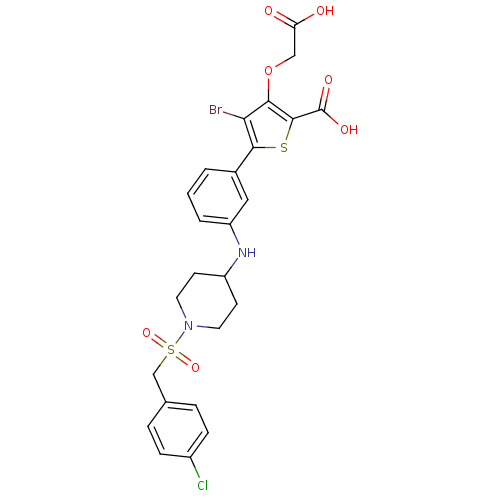
(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(4-chlorobenz...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccc(Cl)cc2)c1 Show InChI InChI=1S/C25H24BrClN2O7S2/c26-21-22(36-13-20(30)31)24(25(32)33)37-23(21)16-2-1-3-19(12-16)28-18-8-10-29(11-9-18)38(34,35)14-15-4-6-17(27)7-5-15/h1-7,12,18,28H,8-11,13-14H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219583
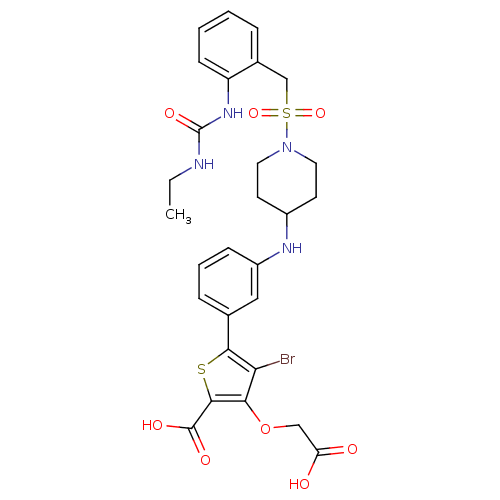
(4-bromo-3-(carboxymethoxy)-5-[3-({1-[(2-{[(ethylam...)Show SMILES CCNC(=O)Nc1ccccc1CS(=O)(=O)N1CCC(CC1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C28H31BrN4O8S2/c1-2-30-28(38)32-21-9-4-3-6-18(21)16-43(39,40)33-12-10-19(11-13-33)31-20-8-5-7-17(14-20)25-23(29)24(41-15-22(34)35)26(42-25)27(36)37/h3-9,14,19,31H,2,10-13,15-16H2,1H3,(H,34,35)(H,36,37)(H2,30,32,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183060
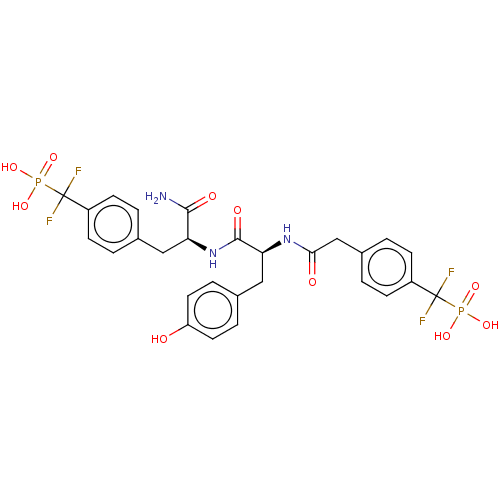
(CHEMBL3818452)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C28H29F4N3O10P2/c29-27(30,46(40,41)42)19-7-1-16(2-8-19)13-22(25(33)38)35-26(39)23(14-17-5-11-21(36)12-6-17)34-24(37)15-18-3-9-20(10-4-18)28(31,32)47(43,44)45/h1-12,22-23,36H,13-15H2,(H2,33,38)(H,34,37)(H,35,39)(H2,40,41,42)(H2,43,44,45)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131550
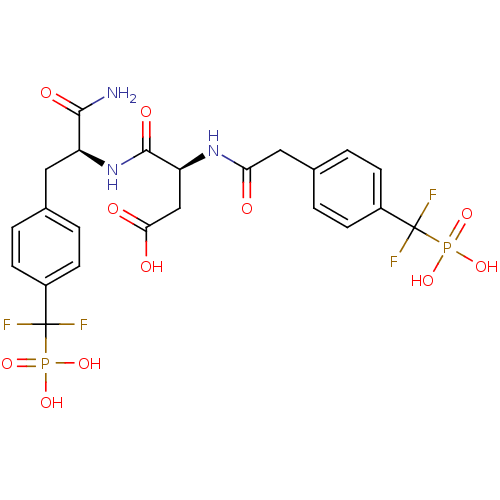
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem Lett 29: 2358-2363 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.011
BindingDB Entry DOI: 10.7270/Q2280C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem 18: 1773-82 (2010)
Article DOI: 10.1016/j.bmc.2010.01.055
BindingDB Entry DOI: 10.7270/Q2X06811 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219573
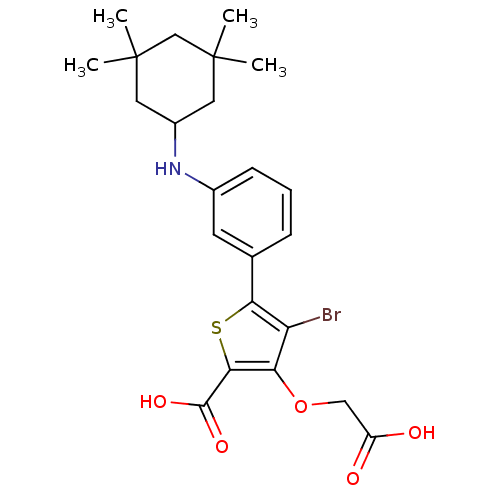
(4-BROMO-3-(CARBOXYMETHOXY)-5-{3-[(3,3,5,5-TETRAMET...)Show SMILES CC1(C)CC(CC(C)(C)C1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C23H28BrNO5S/c1-22(2)9-15(10-23(3,4)12-22)25-14-7-5-6-13(8-14)19-17(24)18(30-11-16(26)27)20(31-19)21(28)29/h5-8,15,25H,9-12H2,1-4H3,(H,26,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP |
J Med Chem 50: 4681-98 (2007)
Article DOI: 10.1021/jm0702478
BindingDB Entry DOI: 10.7270/Q2TX3F37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, PR China; China State Institute of Pharmaceutical Industry, Novel Technology Center of Pharmaceutical Chemistry, Shanghai Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TCPTP (unknown origin) using pNPP as substrate measured after 30 mins |
Bioorg Med Chem Lett 27: 2166-2170 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.060
BindingDB Entry DOI: 10.7270/Q2FR0024 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50219573

(4-BROMO-3-(CARBOXYMETHOXY)-5-{3-[(3,3,5,5-TETRAMET...)Show SMILES CC1(C)CC(CC(C)(C)C1)Nc1cccc(c1)-c1sc(C(O)=O)c(OCC(O)=O)c1Br Show InChI InChI=1S/C23H28BrNO5S/c1-22(2)9-15(10-23(3,4)12-22)25-14-7-5-6-13(8-14)19-17(24)18(30-11-16(26)27)20(31-19)21(28)29/h5-8,15,25H,9-12H2,1-4H3,(H,26,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13954

(3-({5-[(2S)-3-{4-[(2-carboxyphenyl)amidoformic aci...)Show SMILES CC(=O)N[C@@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 49 | -9.86 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
J Am Chem Soc 125: 4087-96 (2003)
Article DOI: 10.1021/ja0296733
BindingDB Entry DOI: 10.7270/Q2B856CS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50149232
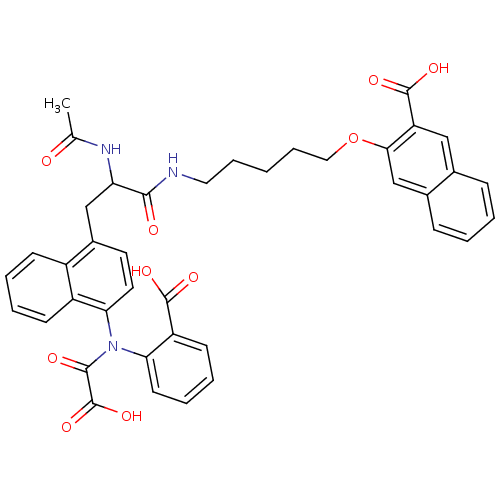
(3-(5-(2-acetamido-3-(4-(carboxy-N-(2-carboxyphenyl...)Show SMILES CC(=O)NC(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 16: 7399-409 (2008)
Article DOI: 10.1016/j.bmc.2008.06.014
BindingDB Entry DOI: 10.7270/Q2PC325T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308852

((S)-2-(N-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methox...)Show SMILES CCc1cc(C[C@H](NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50326165
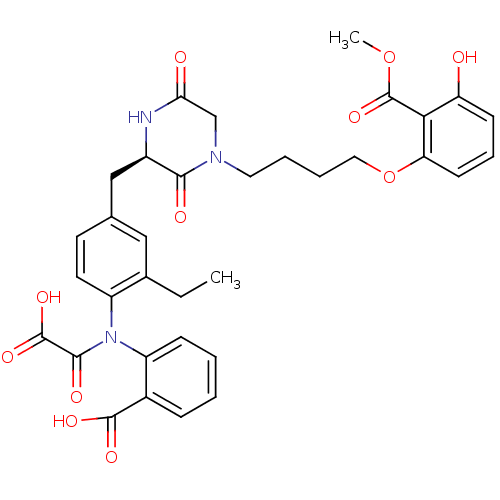
(CHEMBL1241315 | oxalylaminobenzoic acid)Show SMILES CCc1cc(C[C@H]2NC(=O)CN(CCCCOc3cccc(O)c3C(=O)OC)C2=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C34H35N3O11/c1-3-21-17-20(13-14-24(21)37(31(41)33(44)45)25-10-5-4-9-22(25)32(42)43)18-23-30(40)36(19-28(39)35-23)15-6-7-16-48-27-12-8-11-26(38)29(27)34(46)47-2/h4-5,8-14,17,23,38H,3,6-7,15-16,18-19H2,1-2H3,(H,35,39)(H,42,43)(H,44,45)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry |
Eur J Med Chem 45: 3709-18 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.020
BindingDB Entry DOI: 10.7270/Q24Q7V6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13976
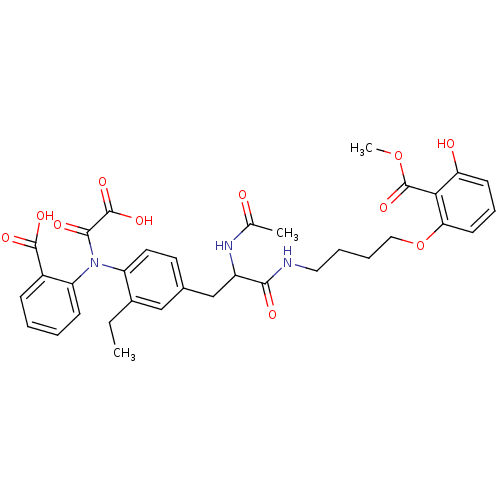
(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Eur J Med Chem 44: 3147-57 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.009
BindingDB Entry DOI: 10.7270/Q2FJ2GTG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14267
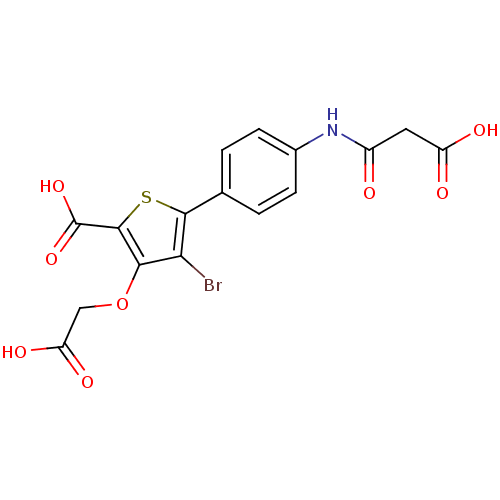
(4-bromo-3-(carboxymethoxy)-5-[4-(2-formamidoacetic...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)CC(O)=O)cc1 Show InChI InChI=1S/C16H12BrNO8S/c17-12-13(26-6-11(22)23)15(16(24)25)27-14(12)7-1-3-8(4-2-7)18-9(19)5-10(20)21/h1-4H,5-6H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13975
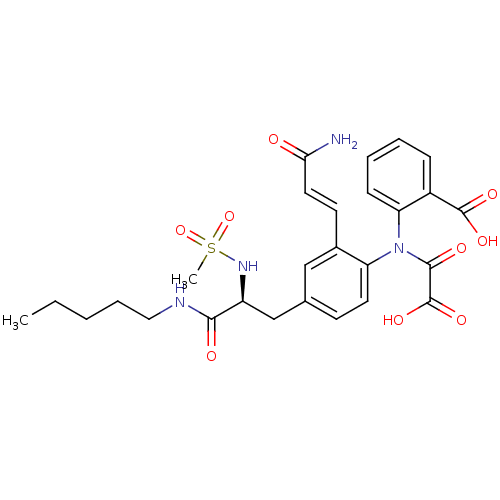
(2-({2-[(1E)-2-carbamoyleth-1-en-1-yl]-4-[(2S)-2-me...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c(\C=C\C(N)=O)c1)NS(C)(=O)=O |r| Show InChI InChI=1S/C27H32N4O9S/c1-3-4-7-14-29-24(33)20(30-41(2,39)40)16-17-10-12-21(18(15-17)11-13-23(28)32)31(25(34)27(37)38)22-9-6-5-8-19(22)26(35)36/h5-6,8-13,15,20,30H,3-4,7,14,16H2,1-2H3,(H2,28,32)(H,29,33)(H,35,36)(H,37,38)/b13-11+/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
J Med Chem 46: 2093-103 (2003)
Article DOI: 10.1021/jm0205696
BindingDB Entry DOI: 10.7270/Q26H4FPH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183059

(CHEMBL3818451)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(C(=O)C(=O)Nc2ccccc2C(O)=O)c(\C=C\C(N)=O)c1)NS(C)(=O)=O |r| Show InChI InChI=1S/C27H32N4O8S/c1-3-4-7-14-29-25(34)22(31-40(2,38)39)16-17-10-12-19(18(15-17)11-13-23(28)32)24(33)26(35)30-21-9-6-5-8-20(21)27(36)37/h5-6,8-13,15,22,31H,3-4,7,14,16H2,1-2H3,(H2,28,32)(H,29,34)(H,30,35)(H,36,37)/b13-11+/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15812

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](Cc2ccccc2)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C36H40N4O10/c1-3-25-19-24(16-17-29(25)40(33(44)36(49)50)30-14-8-7-13-26(30)34(45)46)21-27(38-22(2)41)32(43)37-18-10-9-15-31(42)39-28(35(47)48)20-23-11-5-4-6-12-23/h4-8,11-14,16-17,19,27-28H,3,9-10,15,18,20-21H2,1-2H3,(H,37,43)(H,38,41)(H,39,42)(H,45,46)(H,47,48)(H,49,50)/t27?,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14267

(4-bromo-3-(carboxymethoxy)-5-[4-(2-formamidoacetic...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)CC(O)=O)cc1 Show InChI InChI=1S/C16H12BrNO8S/c17-12-13(26-6-11(22)23)15(16(24)25)27-14(12)7-1-3-8(4-2-7)18-9(19)5-10(20)21/h1-4H,5-6H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | -9.19 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research
| Assay Description
The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... |
Bioorg Med Chem Lett 16: 4941-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.051
BindingDB Entry DOI: 10.7270/Q24J0CBD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15817
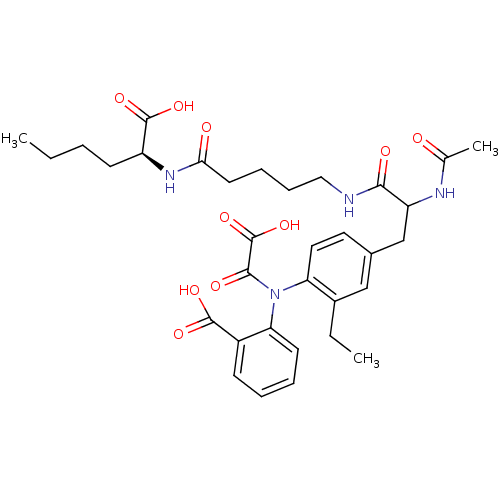
(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCCC[C@H](NC(=O)CCCCNC(=O)C(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c(CC)c1)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C33H42N4O10/c1-4-6-12-24(32(44)45)36-28(39)14-9-10-17-34-29(40)25(35-20(3)38)19-21-15-16-26(22(5-2)18-21)37(30(41)33(46)47)27-13-8-7-11-23(27)31(42)43/h7-8,11,13,15-16,18,24-25H,4-6,9-10,12,14,17,19H2,1-3H3,(H,34,40)(H,35,38)(H,36,39)(H,42,43)(H,44,45)(H,46,47)/t24-,25?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15815
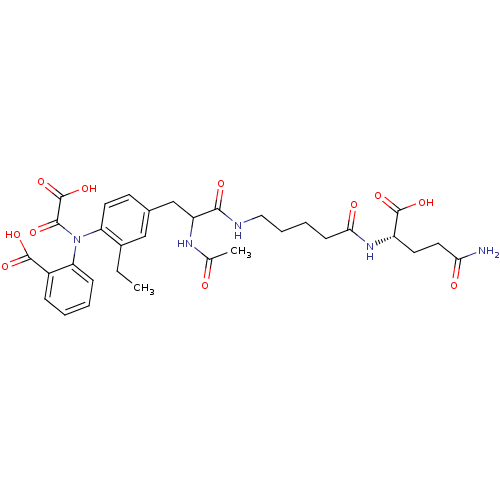
(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCC(N)=O)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H39N5O11/c1-3-20-16-19(11-13-24(20)37(29(42)32(47)48)25-9-5-4-8-21(25)30(43)44)17-23(35-18(2)38)28(41)34-15-7-6-10-27(40)36-22(31(45)46)12-14-26(33)39/h4-5,8-9,11,13,16,22-23H,3,6-7,10,12,14-15,17H2,1-2H3,(H2,33,39)(H,34,41)(H,35,38)(H,36,40)(H,43,44)(H,45,46)(H,47,48)/t22-,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118792
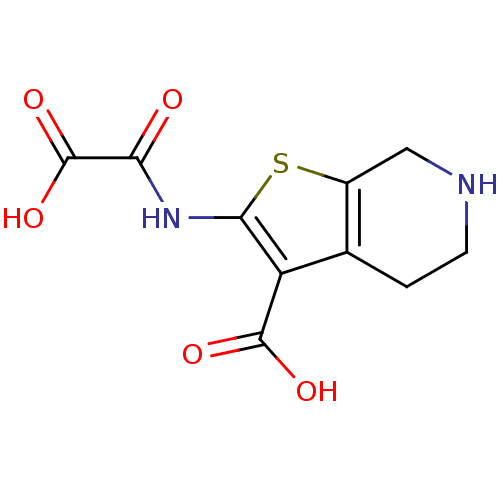
(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15813

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(S)-...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](C2CCCCC2)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C35H44N4O10/c1-3-23-19-22(16-17-27(23)39(32(43)35(48)49)28-14-8-7-13-25(28)33(44)45)20-26(37-21(2)40)31(42)36-18-10-9-15-29(41)38-30(34(46)47)24-11-5-4-6-12-24/h7-8,13-14,16-17,19,24,26,30H,3-6,9-12,15,18,20H2,1-2H3,(H,36,42)(H,37,40)(H,38,41)(H,44,45)(H,46,47)(H,48,49)/t26?,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14245
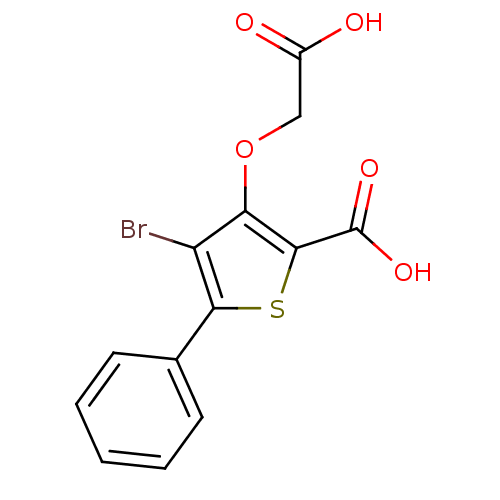
(4-bromo-3-(carboxymethoxy)-5-phenylthiophene-2-car...)Show InChI InChI=1S/C13H9BrO5S/c14-9-10(19-6-8(15)16)12(13(17)18)20-11(9)7-4-2-1-3-5-7/h1-5H,6H2,(H,15,16)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 320 | -8.85 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research
| Assay Description
The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... |
Bioorg Med Chem Lett 16: 4941-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.051
BindingDB Entry DOI: 10.7270/Q24J0CBD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15820
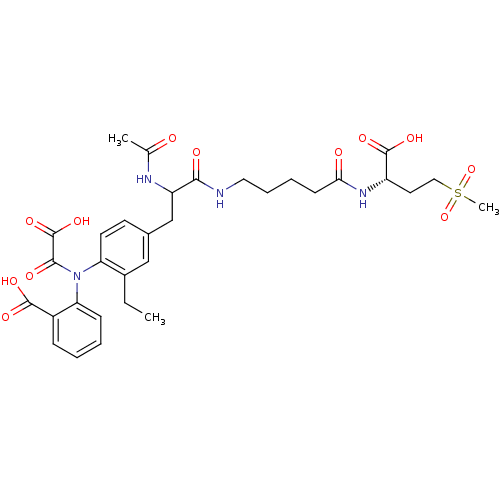
(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCS(C)(=O)=O)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H40N4O12S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-49(3,47)48/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46)/t23-,24?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM14239
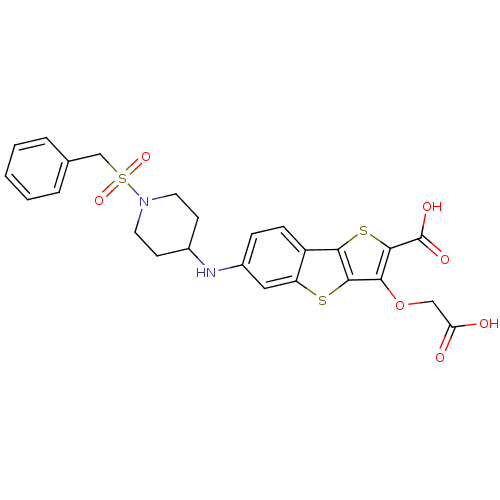
(5-(carboxymethoxy)-10-{[1-(phenylmethane)sulfonylp...)Show SMILES OC(=O)COc1c(sc2c1sc1cc(NC3CCN(CC3)S(=O)(=O)Cc3ccccc3)ccc21)C(O)=O Show InChI InChI=1S/C25H24N2O7S3/c28-20(29)13-34-21-23-22(36-24(21)25(30)31)18-7-6-17(12-19(18)35-23)26-16-8-10-27(11-9-16)37(32,33)14-15-4-2-1-3-5-15/h1-7,12,16,26H,8-11,13-14H2,(H,28,29)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | -8.75 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research
| Assay Description
The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... |
Bioorg Med Chem 14: 2162-77 (2006)
Article DOI: 10.1016/j.bmc.2005.11.005
BindingDB Entry DOI: 10.7270/Q289143K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15819

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46)/t23-,24?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using pNPP as substrate by colorimetric method |
Bioorg Med Chem Lett 29: 2358-2363 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.011
BindingDB Entry DOI: 10.7270/Q2280C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183057
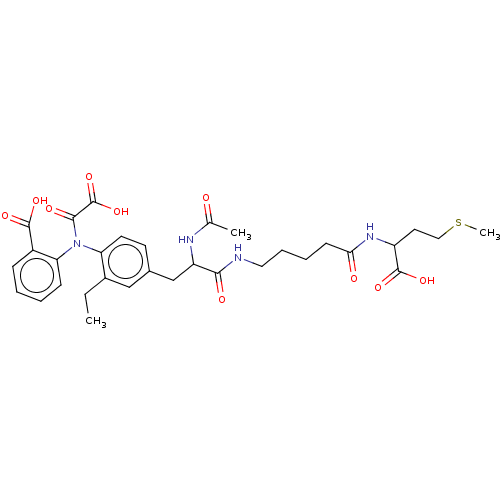
(CHEMBL3818765)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)NC(CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15819

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46)/t23-,24?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15814
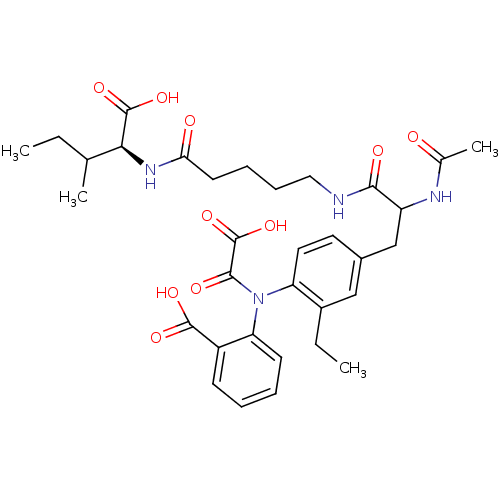
(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCC(C)[C@H](NC(=O)CCCCNC(=O)C(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c(CC)c1)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C33H42N4O10/c1-5-19(3)28(32(44)45)36-27(39)13-9-10-16-34-29(40)24(35-20(4)38)18-21-14-15-25(22(6-2)17-21)37(30(41)33(46)47)26-12-8-7-11-23(26)31(42)43/h7-8,11-12,14-15,17,19,24,28H,5-6,9-10,13,16,18H2,1-4H3,(H,34,40)(H,35,38)(H,36,39)(H,42,43)(H,44,45)(H,46,47)/t19?,24?,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15806
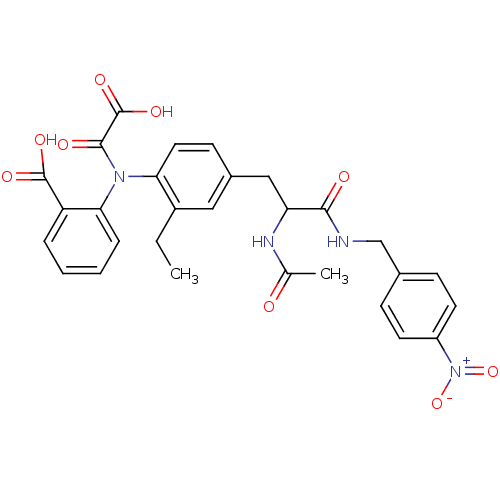
(1:1 racemic mixture | 2-{[4-(2-acetamido-2-{[(4-ni...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCc2ccc(cc2)[N+]([O-])=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N4O9/c1-3-20-14-19(10-13-24(20)32(27(36)29(39)40)25-7-5-4-6-22(25)28(37)38)15-23(31-17(2)34)26(35)30-16-18-8-11-21(12-9-18)33(41)42/h4-14,23H,3,15-16H2,1-2H3,(H,30,35)(H,31,34)(H,37,38)(H,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131545
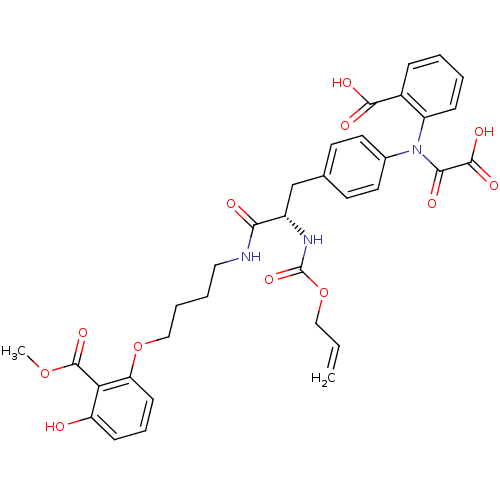
((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM15818
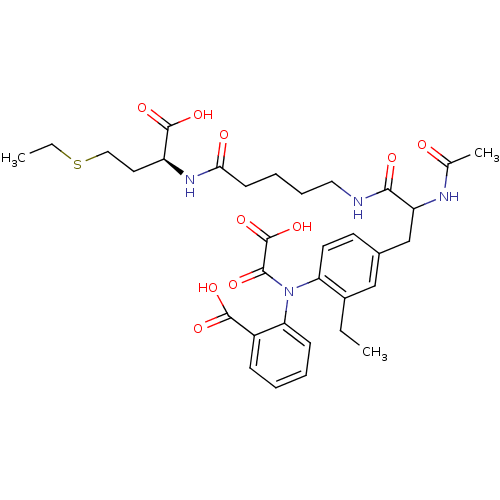
(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCSCC[C@H](NC(=O)CCCCNC(=O)C(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c(CC)c1)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C33H42N4O10S/c1-4-22-18-21(13-14-26(22)37(30(41)33(46)47)27-11-7-6-10-23(27)31(42)43)19-25(35-20(3)38)29(40)34-16-9-8-12-28(39)36-24(32(44)45)15-17-48-5-2/h6-7,10-11,13-14,18,24-25H,4-5,8-9,12,15-17,19H2,1-3H3,(H,34,40)(H,35,38)(H,36,39)(H,42,43)(H,44,45)(H,46,47)/t24-,25?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
Bioorg Med Chem Lett 13: 1887-90 (2003)
Article DOI: 10.1016/S0960-894X(03)00302-0
BindingDB Entry DOI: 10.7270/Q29S1P91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50299461
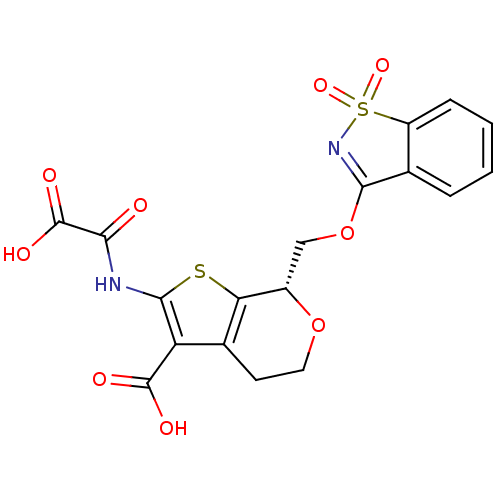
((S)-7-(1,1-Dioxo-1H-1lambda*6*-benzo[d]isothiazol-...)Show SMILES OC(=O)C(=O)Nc1sc2[C@H](COC3=NS(=O)(=O)c4ccccc34)OCCc2c1C(O)=O |r,t:12| Show InChI InChI=1S/C18H14N2O9S2/c21-14(18(24)25)19-16-12(17(22)23)9-5-6-28-10(13(9)30-16)7-29-15-8-3-1-2-4-11(8)31(26,27)20-15/h1-4,10H,5-7H2,(H,19,21)(H,22,23)(H,24,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data