 Found 52 hits of kd for UniProtKB: Q09472
Found 52 hits of kd for UniProtKB: Q09472 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50241990

(CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H](-[#6][C@@]12[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:40:39:26.24.37:15.8.9| Show InChI InChI=1S/C38H50O6/c1-22(2)11-13-27(25(7)8)20-37-21-28(15-12-23(3)4)36(9,10)38(35(37)44,18-17-24(5)6)34(43)31(33(37)42)32(41)26-14-16-29(39)30(40)19-26/h11-12,14,16-17,19,27-28,31,39-40H,7,13,15,18,20-21H2,1-6,8-10H3/t27-,28+,31?,37+,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM321424
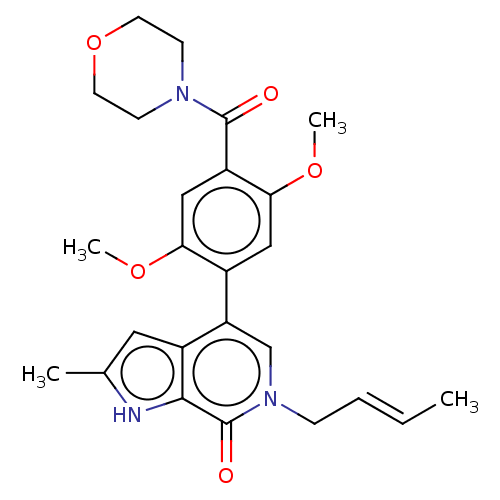
(6-[(E)-but-2-enyl]-4-[2,5-dimethoxy-4-(morpholine-...)Show SMILES COc1cc(c(OC)cc1C(=O)N1CCOCC1)-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12 Show InChI InChI=1S/C25H29N3O5/c1-5-6-7-28-15-20(18-12-16(2)26-23(18)25(28)30)17-13-22(32-4)19(14-21(17)31-3)24(29)27-8-10-33-11-9-27/h5-6,12-15,26H,7-11H2,1-4H3/b6-5+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84874

(Guttiferone A, 3)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@H]1-[#6][C@@]2([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6](=O)[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2=O)[C@]1([#6])[#6]-[#6]=[#6](-[#6])-[#6] |r,THB:35:34:15.26.13:36.6.5| Show InChI InChI=1S/C37H48O6/c1-22(2)10-12-27-21-36(18-15-24(5)6)32(41)30(31(40)26-11-13-28(38)29(39)20-26)33(42)37(34(36)43,19-16-25(7)8)35(27,9)17-14-23(3)4/h10-11,13-16,20,27,30,38-39H,12,17-19,21H2,1-9H3/t27-,30?,35+,36-,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84875
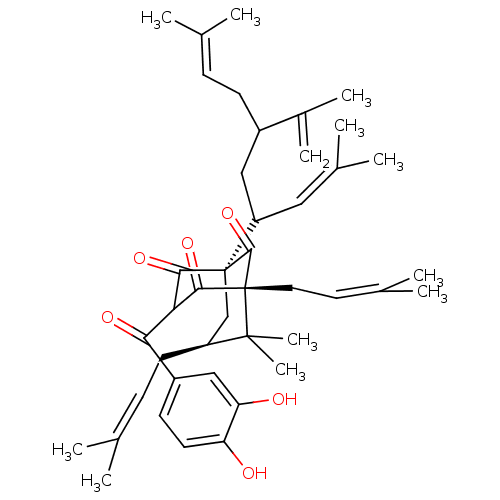
(Guttiferone E, 4)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6](-[#6]-[#6](-[#6]=[#6](-[#6])-[#6])[C@]12[#6]-[#6@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:45:44:31.29.42:20.13.14| Show InChI InChI=1S/C43H58O6/c1-25(2)13-15-30(29(9)10)22-33(21-28(7)8)42-24-32(17-14-26(3)4)41(11,12)43(40(42)49,20-19-27(5)6)39(48)36(38(42)47)37(46)31-16-18-34(44)35(45)23-31/h13-14,16,18-19,21,23,30,32-33,36,44-45H,9,15,17,20,22,24H2,1-8,10-12H3/t30?,32-,33?,36?,42+,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM84877
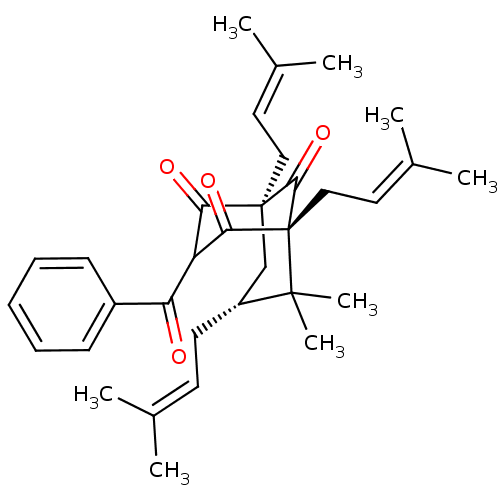
(Clusianone, 10)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H]1-[#6][C@@]2([#6]\[#6]=[#6](/[#6])-[#6])[#6](=O)-[#6](-[#6](=O)-c3ccccc3)-[#6](=O)[C@]([#6]\[#6]=[#6](/[#6])-[#6])([#6]2=O)C1([#6])[#6] |r,THB:33:32:15.24.13:34.6.5| Show InChI InChI=1S/C33H42O4/c1-21(2)14-15-25-20-32(18-16-22(3)4)28(35)26(27(34)24-12-10-9-11-13-24)29(36)33(30(32)37,31(25,7)8)19-17-23(5)6/h9-14,16-17,25-26H,15,18-20H2,1-8H3/t25-,26?,32+,33-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno
| Assay Description
The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... |
Chembiochem 11: 818-27 (2010)
Article DOI: 10.1002/cbic.200900721
BindingDB Entry DOI: 10.7270/Q2S1810J |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081171
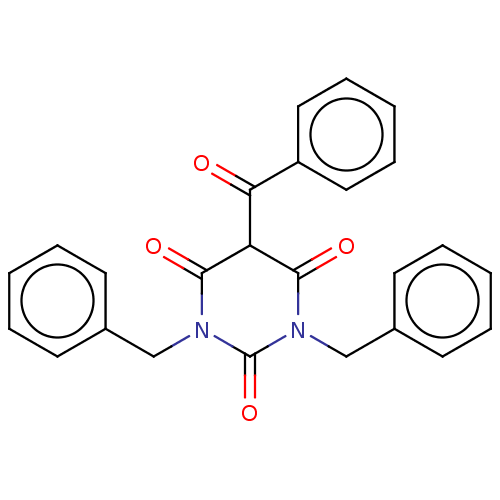
(CHEMBL3421905)Show SMILES O=C(C1C(=O)N(Cc2ccccc2)C(=O)N(Cc2ccccc2)C1=O)c1ccccc1 Show InChI InChI=1S/C25H20N2O4/c28-22(20-14-8-3-9-15-20)21-23(29)26(16-18-10-4-1-5-11-18)25(31)27(24(21)30)17-19-12-6-2-7-13-19/h1-15,21H,16-17H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant P300 catalytic domain by SPR analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081172
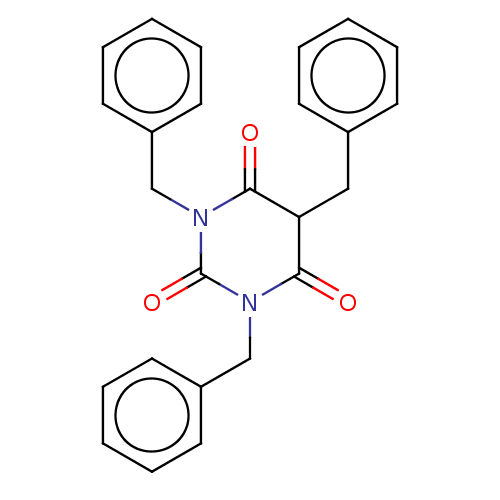
(CHEMBL3421930)Show SMILES O=C1C(Cc2ccccc2)C(=O)N(Cc2ccccc2)C(=O)N1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O3/c28-23-22(16-19-10-4-1-5-11-19)24(29)27(18-21-14-8-3-9-15-21)25(30)26(23)17-20-12-6-2-7-13-20/h1-15,22H,16-18H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant P300 catalytic domain by SPR analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM188519
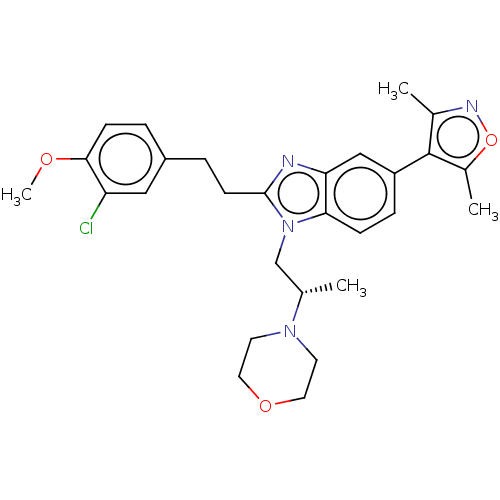
(SGC-CBP30)Show SMILES COc1ccc(CCc2nc3cc(ccc3n2C[C@H](C)N2CCOCC2)-c2c(C)noc2C)cc1Cl |r| Show InChI InChI=1S/C28H33ClN4O3/c1-18(32-11-13-35-14-12-32)17-33-25-8-7-22(28-19(2)31-36-20(28)3)16-24(25)30-27(33)10-6-21-5-9-26(34-4)23(29)15-21/h5,7-9,15-16,18H,6,10-14,17H2,1-4H3/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Binding affinity to human p300 by ITC analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151663

(CHEMBL3774655)Show SMILES CCC(=O)N1CCOc2c(C1)cc(cc2OC[C@H]1CCCN(C)C1)-c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C27H36N2O5/c1-5-26(30)29-11-12-33-27-22(17-29)13-21(20-8-9-23(31-3)24(14-20)32-4)15-25(27)34-18-19-7-6-10-28(2)16-19/h8-9,13-15,19H,5-7,10-12,16-18H2,1-4H3/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a |
University of Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of human p300 expressed in competent escherichia coli BL21(DE3)-R3-pRARE2 cells by isothermal titration calorimetry |
J Med Chem 59: 1249-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01502
BindingDB Entry DOI: 10.7270/Q2DV1MRN |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50396040
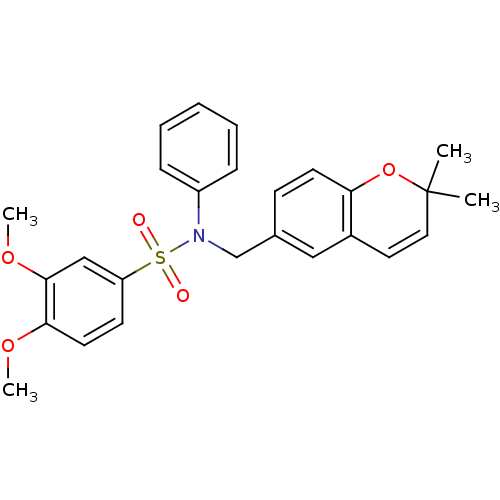
(CHEMBL1823895)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N(Cc1ccc2OC(C)(C)C=Cc2c1)c1ccccc1 |c:24| Show InChI InChI=1S/C26H27NO5S/c1-26(2)15-14-20-16-19(10-12-23(20)32-26)18-27(21-8-6-5-7-9-21)33(28,29)22-11-13-24(30-3)25(17-22)31-4/h5-17H,18H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant P300 CH1 domain assessed as dissociation constant by surface plasmon resonance analysis |
ACS Med Chem Lett 3: 620-625 (2012)
Article DOI: 10.1021/ml300042k
BindingDB Entry DOI: 10.7270/Q2K64K5R |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50241990

(CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H](-[#6][C@@]12[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:40:39:26.24.37:15.8.9| Show InChI InChI=1S/C38H50O6/c1-22(2)11-13-27(25(7)8)20-37-21-28(15-12-23(3)4)36(9,10)38(35(37)44,18-17-24(5)6)34(43)31(33(37)42)32(41)26-14-16-29(39)30(40)19-26/h11-12,14,16-17,19,27-28,31,39-40H,7,13,15,18,20-21H2,1-6,8-10H3/t27-,28+,31?,37+,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Binding affinity to HAT p300 catalytic domain by fluorometric titration |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50265449

((1R,3S,9S,11R)-7-(3-Hydroxy-4-methoxy-benzoyl)-4,4...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2)[#6]-1=O |r,c:12| Show InChI InChI=1S/C39H54O5/c1-24(2)12-15-28-21-38-22-29(16-13-25(3)4)37(9,10)44-35(38)32(33(41)27-14-17-31(43-11)30(40)20-27)34(42)39(23-38,36(28,7)8)19-18-26(5)6/h12-14,17-18,20,28-29,40H,15-16,19,21-23H2,1-11H3/t28-,29+,38-,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Binding affinity to HAT p300 catalytic domain by fluorometric titration |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50377962
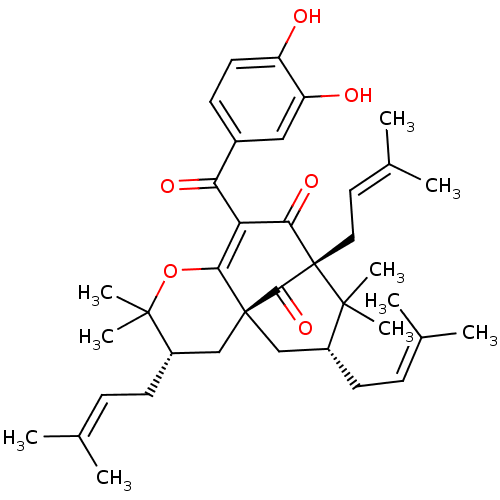
(CAMBOGIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6@H]1-[#6][C@@]23[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c4ccc(-[#8])c(-[#8])c4)=[#6]2-[#8]C1([#6])[#6])[#6]3=O |r,c:37,TLB:43:42:15.8.9:26.24.37| Show InChI InChI=1S/C38H50O6/c1-22(2)11-14-26-20-37-21-27(15-12-23(3)4)36(9,10)44-33(37)30(31(41)25-13-16-28(39)29(40)19-25)32(42)38(34(37)43,35(26,7)8)18-17-24(5)6/h11-13,16-17,19,26-27,39-40H,14-15,18,20-21H2,1-10H3/t26-,27+,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Binding affinity to HAT p300 catalytic domain by fluorometric titration |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50241990

(CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H](-[#6][C@@]12[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:40:39:26.24.37:15.8.9| Show InChI InChI=1S/C38H50O6/c1-22(2)11-13-27(25(7)8)20-37-21-28(15-12-23(3)4)36(9,10)38(35(37)44,18-17-24(5)6)34(43)31(33(37)42)32(41)26-14-16-29(39)30(40)19-26/h11-12,14,16-17,19,27-28,31,39-40H,7,13,15,18,20-21H2,1-6,8-10H3/t27-,28+,31?,37+,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Binding affinity to HAT p300 catalytic domain by isothermal titration calorimetry |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50265449

((1R,3S,9S,11R)-7-(3-Hydroxy-4-methoxy-benzoyl)-4,4...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2)[#6]-1=O |r,c:12| Show InChI InChI=1S/C39H54O5/c1-24(2)12-15-28-21-38-22-29(16-13-25(3)4)37(9,10)44-35(38)32(33(41)27-14-17-31(43-11)30(40)20-27)34(42)39(23-38,36(28,7)8)19-18-26(5)6/h12-14,17-18,20,28-29,40H,15-16,19,21-23H2,1-11H3/t28-,29+,38-,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Binding affinity to HAT p300 catalytic domain by isothermal titration calorimetry |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50377962

(CAMBOGIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6@H]1-[#6][C@@]23[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c4ccc(-[#8])c(-[#8])c4)=[#6]2-[#8]C1([#6])[#6])[#6]3=O |r,c:37,TLB:43:42:15.8.9:26.24.37| Show InChI InChI=1S/C38H50O6/c1-22(2)11-14-26-20-37-21-27(15-12-23(3)4)36(9,10)44-33(37)30(31(41)25-13-16-28(39)29(40)19-25)32(42)38(34(37)43,35(26,7)8)18-17-24(5)6/h11-13,16-17,19,26-27,39-40H,14-15,18,20-21H2,1-10H3/t26-,27+,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Binding affinity to HAT p300 catalytic domain by isothermal titration calorimetry |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM220447

(US10633379, Compound X | US9296741, 36)Show SMILES CCS(=O)(=O)Nc1ccc(Oc2ccc(F)cc2F)c(c1)-c1cn(C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H19F2N3O4S/c1-3-32(29,30)26-14-5-7-19(31-20-6-4-13(23)10-18(20)24)16(11-14)17-12-27(2)22(28)21-15(17)8-9-25-21/h4-12,25-26H,3H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 60: 8369-8384 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00746
BindingDB Entry DOI: 10.7270/Q2251MB8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50366670
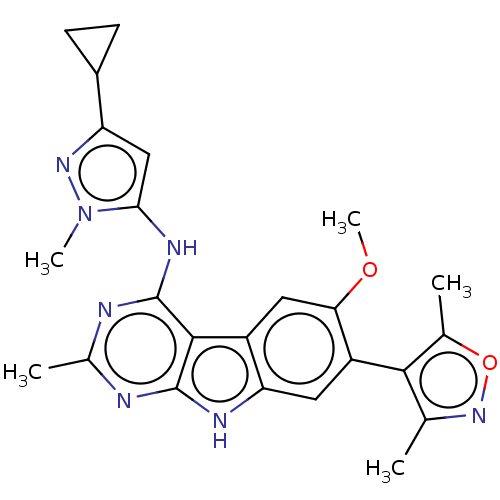
(CHEMBL4173488)Show SMILES COc1cc2c(cc1-c1c(C)noc1C)[nH]c1nc(C)nc(Nc3cc(nn3C)C3CC3)c21 |(86.87,-46.33,;85.61,-45.43,;84.21,-46.07,;84.06,-47.6,;82.66,-48.24,;81.41,-47.34,;81.56,-45.82,;82.95,-45.18,;83.09,-43.65,;81.94,-42.63,;80.43,-42.97,;82.55,-41.22,;84.08,-41.36,;84.42,-42.87,;85.83,-43.48,;80.17,-48.26,;80.66,-49.73,;79.89,-51.06,;80.66,-52.39,;79.89,-53.73,;82.21,-52.4,;82.97,-51.06,;84.51,-51.06,;85.28,-52.39,;84.66,-53.8,;85.81,-54.83,;87.14,-54.06,;86.82,-52.56,;87.85,-51.41,;85.64,-56.36,;84.75,-57.61,;86.28,-57.77,;82.2,-49.71,)| Show InChI InChI=1S/C24H25N7O2/c1-11-21(12(2)33-30-11)16-8-18-15(9-19(16)32-5)22-23(27-18)25-13(3)26-24(22)28-20-10-17(14-6-7-14)29-31(20)4/h8-10,14H,6-7H2,1-5H3,(H2,25,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOScan assay |
J Med Chem 61: 6110-6120 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00483
BindingDB Entry DOI: 10.7270/Q26M39B1 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50455480
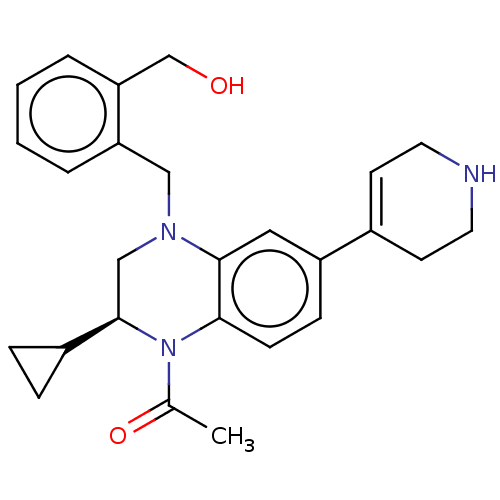
(CHEMBL4218735)Show SMILES CC(=O)N1[C@H](CN(Cc2ccccc2CO)c2cc(ccc12)C1=CCNCC1)C1CC1 |r,t:25| Show InChI InChI=1S/C26H31N3O2/c1-18(31)29-24-9-8-21(19-10-12-27-13-11-19)14-25(24)28(16-26(29)20-6-7-20)15-22-4-2-3-5-23(22)17-30/h2-5,8-10,14,20,26-27,30H,6-7,11-13,15-17H2,1H3/t26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | <3.02E+4 | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length DNA-tagged EP300 expressed in bacteria by BROMOscan method |
J Med Chem 61: 4317-4334 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01666
BindingDB Entry DOI: 10.7270/Q27H1N6W |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM179481
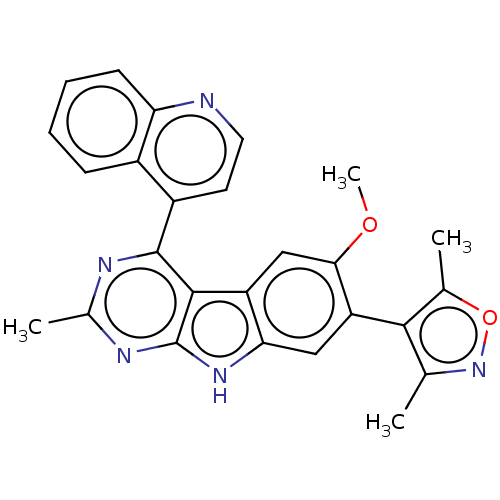
(US9675697, Cpd. No. 73)Show SMILES COc1cc2c(cc1-c1c(C)noc1C)[nH]c1nc(C)nc(-c3ccnc4ccccc34)c21 |(-1.5,3.22,;-2.13,1.81,;-1.36,.48,;.18,.48,;.95,-.85,;.18,-2.19,;-1.36,-2.19,;-2.13,-.85,;-3.67,-.85,;-4.57,-2.1,;-4.18,-3.59,;-6.04,-1.62,;-6.04,-.08,;-4.57,.39,;-4.18,1.88,;1.21,-3.33,;2.62,-2.71,;4.03,-3.33,;5.27,-2.43,;6.68,-3.05,;5.11,-.9,;3.7,-.27,;3.31,1.22,;1.84,1.69,;1.52,3.2,;2.66,4.23,;4.13,3.75,;5.27,4.79,;6.74,4.31,;7.06,2.8,;5.91,1.77,;4.45,2.25,;2.46,-1.17,)| Show InChI InChI=1S/C26H21N5O2/c1-13-23(14(2)33-31-13)19-11-21-18(12-22(19)32-4)24-25(28-15(3)29-26(24)30-21)17-9-10-27-20-8-6-5-7-16(17)20/h5-12H,1-4H3,(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 60: 3887-3901 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00193
BindingDB Entry DOI: 10.7270/Q28W3GWN |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50453949

(CHEMBL4208820 | US11247989, Example 87)Show SMILES CNC(=O)c1ccc(cn1)-c1cc2cccc(-c3nn(C4CCOCC4)c4CCN(Cc34)C(C)=O)c2cn1 Show InChI InChI=1S/C29H30N6O3/c1-18(36)34-11-8-27-24(17-34)28(33-35(27)21-9-12-38-13-10-21)22-5-3-4-19-14-26(32-16-23(19)22)20-6-7-25(31-15-20)29(37)30-2/h3-7,14-16,21H,8-13,17H2,1-2H3,(H,30,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOscan assay |
Bioorg Med Chem Lett 28: 15-23 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.025
BindingDB Entry DOI: 10.7270/Q2ZG6VVZ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537291

(CHEMBL4555191)Show SMILES CN1C(=O)[C@@]2(Cc3ccc(C[C@@]45SS[C@@](CO)(N(C)C4=O)C(=O)N5C)cc3)SS[C@]1(CO)C(=O)N2C |r| Show InChI InChI=1S/C22H26N4O6S4/c1-23-17(31)21(11-27)25(3)15(29)19(23,33-35-21)9-13-5-7-14(8-6-13)10-20-16(30)26(4)22(12-28,36-34-20)18(32)24(20)2/h5-8,27-28H,9-12H2,1-4H3/t19-,20-,21-,22-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-fused p300 CH1 domain (323 to 423 residues) under hypoxic condition by SPR assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524839

(CHEMBL4466328)Show SMILES CC(C)C[C@H](N1CCN[C@@H](CC(C)C)C1=O)C(=O)N1CCN([C@@H](CCC(N)=O)C(N)=O)C(=O)[C@@H]1C |r| Show InChI InChI=1S/C24H42N6O5/c1-14(2)12-17-23(34)29(9-8-27-17)19(13-15(3)4)24(35)28-10-11-30(22(33)16(28)5)18(21(26)32)6-7-20(25)31/h14-19,27H,6-13H2,1-5H3,(H2,25,31)(H2,26,32)/t16-,17-,18-,19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 CH1 domain (unknown origin) by tryptophan fluorescence assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537293

(CHEMBL4590644)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C51H79N13O21S/c1-21(2)15-29(57-41(74)27(52)11-13-35(53)68)46(79)64-40(24(6)66)50(83)61-33(19-65)47(80)58-30(16-25-7-9-26(67)10-8-25)44(77)59-32(18-38(72)73)45(78)62-34(20-86)48(81)56-28(12-14-37(70)71)42(75)63-39(22(3)4)49(82)60-31(17-36(54)69)43(76)55-23(5)51(84)85/h7-10,21-24,27-34,39-40,65-67,86H,11-20,52H2,1-6H3,(H2,53,68)(H2,54,69)(H,55,76)(H,56,81)(H,57,74)(H,58,80)(H,59,77)(H,60,82)(H,61,83)(H,62,78)(H,63,75)(H,64,79)(H,70,71)(H,72,73)(H,84,85)/t23-,24+,27-,28-,29-,30-,31-,32-,33-,34-,39-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.74E+3 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 (unknown origin) by fluorescence anisotrophy |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537294

(CHEMBL4514946)Show SMILES CCCC[C@H](N1CCN[C@@H](CC(C)C)C1=O)C(=O)N1CCN([C@@H](CCC(N)=O)C(N)=O)C(=O)[C@@H]1C |r| Show InChI InChI=1S/C24H42N6O5/c1-5-6-7-19(29-11-10-27-17(23(29)34)14-15(2)3)24(35)28-12-13-30(22(33)16(28)4)18(21(26)32)8-9-20(25)31/h15-19,27H,5-14H2,1-4H3,(H2,25,31)(H2,26,32)/t16-,17-,18-,19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 CH1 domain (unknown origin) by tryptophan fluorescence assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537295

(CHEMBL4524475)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)N1C\C=C\CCN[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r,t:23| Show InChI InChI=1S/C42H71N13O13/c1-21(2)17-27-37(64)50-24(6)41(68)55(16-9-7-8-14-47-26(19-32(57)58)36(63)53-27)30(11-10-15-48-42(45)46)40(67)49-23(5)35(62)52-28(18-22(3)4)38(65)54-29(20-33(59)60)39(66)51-25(34(44)61)12-13-31(43)56/h7,9,21-30,47H,8,10-20H2,1-6H3,(H2,43,56)(H2,44,61)(H,49,67)(H,50,64)(H,51,66)(H,52,62)(H,53,63)(H,54,65)(H,57,58)(H,59,60)(H4,45,46,48)/b9-7+/t23-,24-,25-,26-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 CH1 domain (unknown origin) by fluorescence anisotropy competition assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524834
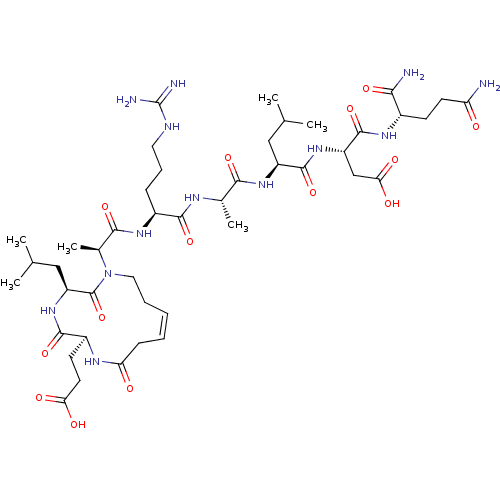
(CHEMBL4435255)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N1CC\C=C/CC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r,c:29| Show InChI InChI=1S/C44H73N13O14/c1-22(2)19-29(41(69)55-30(21-35(62)63)42(70)52-26(36(46)64)13-15-32(45)58)54-37(65)24(5)50-39(67)27(11-10-17-49-44(47)48)53-38(66)25(6)57-18-9-7-8-12-33(59)51-28(14-16-34(60)61)40(68)56-31(43(57)71)20-23(3)4/h7-8,22-31H,9-21H2,1-6H3,(H2,45,58)(H2,46,64)(H,50,67)(H,51,59)(H,52,70)(H,53,66)(H,54,65)(H,55,69)(H,56,68)(H,60,61)(H,62,63)(H4,47,48,49)/b8-7-/t24-,25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 (unknown origin) |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524839

(CHEMBL4466328)Show SMILES CC(C)C[C@H](N1CCN[C@@H](CC(C)C)C1=O)C(=O)N1CCN([C@@H](CCC(N)=O)C(N)=O)C(=O)[C@@H]1C |r| Show InChI InChI=1S/C24H42N6O5/c1-14(2)12-17-23(34)29(9-8-27-17)19(13-15(3)4)24(35)28-10-11-30(22(33)16(28)5)18(21(26)32)6-7-20(25)31/h14-19,27H,6-13H2,1-5H3,(H2,25,31)(H2,26,32)/t16-,17-,18-,19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300-CH1 domain (unknown origin) |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537297

(CHEMBL4542925)Show SMILES CN1C(=O)[C@@]2(Cc3ccc(CCc4ccc(C[C@]56SS[C@](CO)(N(C)C5=O)C(=O)N6C)cc4)cc3)NC(=O)[C@]1(CO)SS2 |r| Show InChI InChI=1S/C29H32N4O6S4/c1-31-23(37)26(30-22(36)28(31,16-34)42-40-26)14-20-10-6-18(7-11-20)4-5-19-8-12-21(13-9-19)15-27-24(38)33(3)29(17-35,43-41-27)25(39)32(27)2/h6-13,34-35H,4-5,14-17H2,1-3H3,(H,30,36)/t26-,27-,28-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-fused p300 CH1 domain (323 to 423 residues) under hypoxic condition by SPR assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537301

(CHEMBL4593074)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O |r| Show InChI InChI=1S/C189H308N54O69S2/c1-25-91(20)148(240-180(304)128-33-28-57-242(128)185(309)93(22)208-164(288)116(68-133(195)254)233-181(305)146(89(16)17)238-163(287)107(45-53-142(266)267)219-178(302)126(81-313)237-174(298)120(73-145(272)273)232-170(294)115(67-95-34-36-96(249)37-35-95)228-177(301)125(80-247)236-184(308)149(94(23)248)241-175(299)114(65-87(12)13)224-158(282)103(40-48-131(193)252)220-179(303)127-32-29-58-243(127)186(310)121(66-88(14)15)210-137(258)75-206-154(278)123(78-245)235-160(284)106(44-52-141(264)265)218-172(296)118(71-143(268)269)230-161(285)108(54-59-314-24)212-151(275)97(190)77-244)183(307)221-101(39-47-130(192)251)153(277)205-76-138(259)211-124(79-246)176(300)214-99(31-27-56-203-189(200)201)156(280)229-117(69-134(196)255)171(295)227-113(64-86(10)11)169(293)226-111(62-84(6)7)166(290)215-100(38-46-129(191)250)152(276)204-74-136(257)209-102(42-50-139(260)261)157(281)216-105(43-51-140(262)263)159(283)223-112(63-85(8)9)168(292)225-110(61-83(4)5)165(289)213-98(30-26-55-202-188(198)199)155(279)207-92(21)150(274)222-109(60-82(2)3)167(291)231-119(72-144(270)271)173(297)217-104(41-49-132(194)253)162(286)239-147(90(18)19)182(306)234-122(187(311)312)70-135(197)256/h34-37,82-94,97-128,146-149,244-249,313H,25-33,38-81,190H2,1-24H3,(H2,191,250)(H2,192,251)(H2,193,252)(H2,194,253)(H2,195,254)(H2,196,255)(H2,197,256)(H,204,276)(H,205,277)(H,206,278)(H,207,279)(H,208,288)(H,209,257)(H,210,258)(H,211,259)(H,212,275)(H,213,289)(H,214,300)(H,215,290)(H,216,281)(H,217,297)(H,218,296)(H,219,302)(H,220,303)(H,221,307)(H,222,274)(H,223,283)(H,224,282)(H,225,292)(H,226,293)(H,227,295)(H,228,301)(H,229,280)(H,230,285)(H,231,291)(H,232,294)(H,233,305)(H,234,306)(H,235,284)(H,236,308)(H,237,298)(H,238,287)(H,239,286)(H,240,304)(H,241,299)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H,268,269)(H,270,271)(H,272,273)(H,311,312)(H4,198,199,202)(H4,200,201,203)/t91-,92-,93-,94+,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,146-,147-,148-,149-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 (unknown origin) by ITC assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50537301

(CHEMBL4593074)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O |r| Show InChI InChI=1S/C189H308N54O69S2/c1-25-91(20)148(240-180(304)128-33-28-57-242(128)185(309)93(22)208-164(288)116(68-133(195)254)233-181(305)146(89(16)17)238-163(287)107(45-53-142(266)267)219-178(302)126(81-313)237-174(298)120(73-145(272)273)232-170(294)115(67-95-34-36-96(249)37-35-95)228-177(301)125(80-247)236-184(308)149(94(23)248)241-175(299)114(65-87(12)13)224-158(282)103(40-48-131(193)252)220-179(303)127-32-29-58-243(127)186(310)121(66-88(14)15)210-137(258)75-206-154(278)123(78-245)235-160(284)106(44-52-141(264)265)218-172(296)118(71-143(268)269)230-161(285)108(54-59-314-24)212-151(275)97(190)77-244)183(307)221-101(39-47-130(192)251)153(277)205-76-138(259)211-124(79-246)176(300)214-99(31-27-56-203-189(200)201)156(280)229-117(69-134(196)255)171(295)227-113(64-86(10)11)169(293)226-111(62-84(6)7)166(290)215-100(38-46-129(191)250)152(276)204-74-136(257)209-102(42-50-139(260)261)157(281)216-105(43-51-140(262)263)159(283)223-112(63-85(8)9)168(292)225-110(61-83(4)5)165(289)213-98(30-26-55-202-188(198)199)155(279)207-92(21)150(274)222-109(60-82(2)3)167(291)231-119(72-144(270)271)173(297)217-104(41-49-132(194)253)162(286)239-147(90(18)19)182(306)234-122(187(311)312)70-135(197)256/h34-37,82-94,97-128,146-149,244-249,313H,25-33,38-81,190H2,1-24H3,(H2,191,250)(H2,192,251)(H2,193,252)(H2,194,253)(H2,195,254)(H2,196,255)(H2,197,256)(H,204,276)(H,205,277)(H,206,278)(H,207,279)(H,208,288)(H,209,257)(H,210,258)(H,211,259)(H,212,275)(H,213,289)(H,214,300)(H,215,290)(H,216,281)(H,217,297)(H,218,296)(H,219,302)(H,220,303)(H,221,307)(H,222,274)(H,223,283)(H,224,282)(H,225,292)(H,226,293)(H,227,295)(H,228,301)(H,229,280)(H,230,285)(H,231,291)(H,232,294)(H,233,305)(H,234,306)(H,235,284)(H,236,308)(H,237,298)(H,238,287)(H,239,286)(H,240,304)(H,241,299)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H,268,269)(H,270,271)(H,272,273)(H,311,312)(H4,198,199,202)(H4,200,201,203)/t91-,92-,93-,94+,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,146-,147-,148-,149-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 (unknown origin) by fluorescence anisotrophy |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
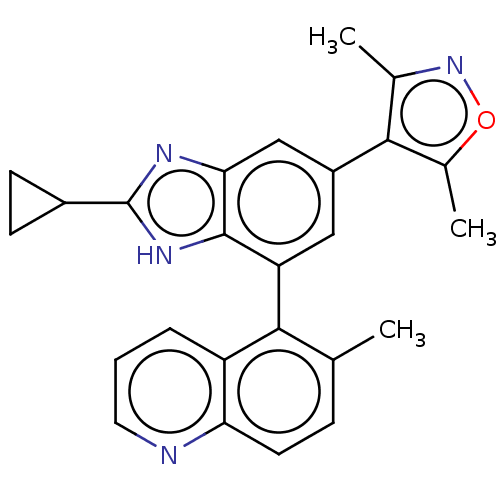
(Homo sapiens (Human)) | BDBM249277
(US10017501, Compound 1020-18 | US9458145, 1020-18)Show SMILES Cc1noc(C)c1-c1cc(-c2c(C)ccc3ncccc23)c2[nH]c(nc2c1)C1CC1 |(1.89,3.57,;.56,4.34,;.08,5.8,;-1.46,5.8,;-1.94,4.34,;-3.27,3.57,;-.69,3.44,;-.69,1.9,;.64,1.13,;.64,-.41,;1.98,-1.18,;3.31,-.41,;3.31,1.13,;4.65,-1.18,;4.65,-2.72,;3.31,-3.49,;3.31,-5.03,;1.98,-5.8,;.64,-5.03,;.64,-3.49,;1.98,-2.72,;-.69,-1.18,;-1.01,-2.69,;-2.54,-2.85,;-3.17,-1.45,;-2.02,-.41,;-2.02,1.13,;-3.31,-4.19,;-4.65,-4.96,;-3.31,-5.73,)| Show InChI InChI=1S/C25H22N4O/c1-13-6-9-20-18(5-4-10-26-20)22(13)19-11-17(23-14(2)29-30-15(23)3)12-21-24(19)28-25(27-21)16-7-8-16/h4-6,9-12,16H,7-8H2,1-3H3,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system after 1 hr by bromoscan assay |
Bioorg Med Chem 27: 457-469 (2019)
Article DOI: 10.1016/j.bmc.2018.11.020
BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
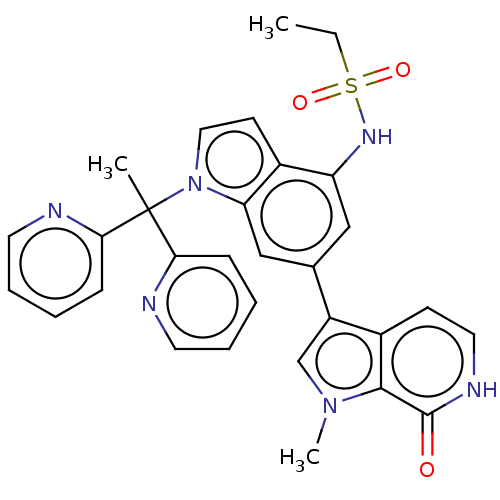
(Homo sapiens (Human)) | BDBM50539801
(CHEMBL4648912 | US11840533, Compound 70)Show SMILES CCS(=O)(=O)Nc1cc(cc2n(ccc12)C(C)(c1ccccn1)c1ccccn1)-c1cn(C)c2c1cc[nH]c2=O Show InChI InChI=1S/C30H28N6O3S/c1-4-40(38,39)34-24-17-20(23-19-35(3)28-21(23)11-15-33-29(28)37)18-25-22(24)12-16-36(25)30(2,26-9-5-7-13-31-26)27-10-6-8-14-32-27/h5-19,34H,4H2,1-3H3,(H,33,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 63: 7186-7210 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00456
BindingDB Entry DOI: 10.7270/Q2PK0KPP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM188519

(SGC-CBP30)Show SMILES COc1ccc(CCc2nc3cc(ccc3n2C[C@H](C)N2CCOCC2)-c2c(C)noc2C)cc1Cl |r| Show InChI InChI=1S/C28H33ClN4O3/c1-18(32-11-13-35-14-12-32)17-33-25-8-7-22(28-19(2)31-36-20(28)3)16-24(25)30-27(33)10-6-21-5-9-26(34-4)23(29)15-21/h5,7-9,15-16,18H,6,10-14,17H2,1-4H3/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.18E+7 | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Reverse ITC (compound as receptor). Domain start/stop: A1040-S1171 |
Proc Natl Acad Sci U S A 112: 10768-73 (2015)
Article DOI: 10.1073/pnas.1501956112
BindingDB Entry DOI: 10.7270/Q2NG4V7B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
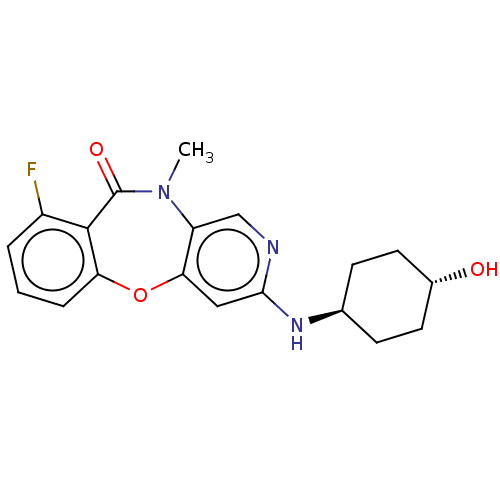
(Homo sapiens (Human)) | BDBM50559467
(CHEMBL4763016)Show SMILES CN1c2cnc(N[C@H]3CC[C@H](O)CC3)cc2Oc2cccc(F)c2C1=O |r,wU:7.6,wD:10.10,(51.72,-32.35,;52.07,-33.85,;50.87,-34.83,;49.54,-34.06,;48.21,-34.83,;48.21,-36.37,;46.88,-37.14,;46.88,-38.67,;48.21,-39.44,;48.21,-40.98,;46.87,-41.74,;46.87,-43.28,;45.55,-40.97,;45.55,-39.44,;49.54,-37.14,;50.88,-36.37,;52.1,-37.32,;53.61,-36.96,;54.5,-38.23,;56.04,-38.09,;56.69,-36.68,;55.8,-35.42,;56.44,-34.03,;54.26,-35.56,;53.59,-34.17,;54.53,-32.96,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOscan method |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116015
BindingDB Entry DOI: 10.7270/Q2TF021T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50365463

(CHEMBL1232461)Show SMILES CCNC(=O)C[C@@H]1N=C(c2ccc(Cl)cc2)c2cc(OC)ccc2-n2c(C)nnc12 |r,t:7| Show InChI InChI=1S/C22H22ClN5O2/c1-4-24-20(29)12-18-22-27-26-13(2)28(22)19-10-9-16(30-3)11-17(19)21(25-18)14-5-7-15(23)8-6-14/h5-11,18H,4,12H2,1-3H3,(H,24,29)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BROMOscan method |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116015
BindingDB Entry DOI: 10.7270/Q2TF021T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
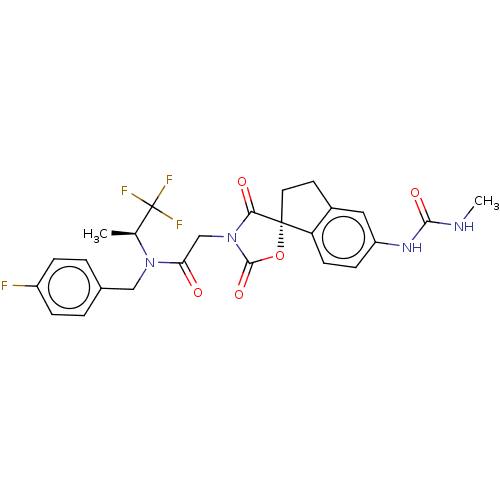
(Homo sapiens (Human)) | BDBM50467836
(CHEMBL4282264)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccc(F)cc3)[C@@H](C)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C25H24F4N4O5/c1-14(25(27,28)29)32(12-15-3-5-17(26)6-4-15)20(34)13-33-21(35)24(38-23(33)37)10-9-16-11-18(7-8-19(16)24)31-22(36)30-2/h3-8,11,14H,9-10,12-13H2,1-2H3,(H2,30,31,36)/t14-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.00128 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human Biotin-Avi-P300 assessed as dissociation constant using Neutravidin as ligand measured by Surface Plasmon Resonance method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00654
BindingDB Entry DOI: 10.7270/Q2N58R45 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50467836

(CHEMBL4282264)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccc(F)cc3)[C@@H](C)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C25H24F4N4O5/c1-14(25(27,28)29)32(12-15-3-5-17(26)6-4-15)20(34)13-33-21(35)24(38-23(33)37)10-9-16-11-18(7-8-19(16)24)31-22(36)30-2/h3-8,11,14H,9-10,12-13H2,1-2H3,(H2,30,31,36)/t14-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human Biotin-Avi-P300 using Neutravidin as ligand measured by Surface Plasmon Resonance method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00654
BindingDB Entry DOI: 10.7270/Q2N58R45 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50159140

(CHEMBL3785648)Show InChI InChI=1S/C11H16N2O2/c1-5-8-9(7(3)14)6(2)13-10(8)11(15)12-4/h13H,5H2,1-4H3,(H,12,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-Tev tagged EP300 expressed in bacterial expression system incubated for 30 mins by isothermal titration calorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112861
BindingDB Entry DOI: 10.7270/Q2CC14D7 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50559314

(CHEMBL4741587)Show SMILES CCc1c([nH]c(C)c1C(C)=O)C(=O)NCc1c(O)ccc2cccc(O)c12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human His-Tev tagged EP300 expressed in bacterial expression system incubated for 30 mins by isothermal titration calorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112861
BindingDB Entry DOI: 10.7270/Q2CC14D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50571550
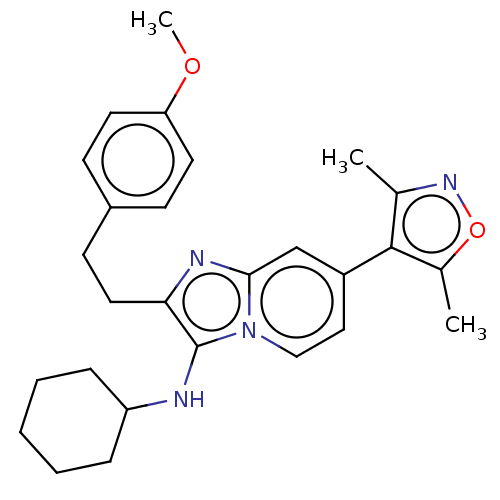
(CHEMBL4867046)Show SMILES COc1ccc(CCc2nc3cc(ccn3c2NC2CCCCC2)-c2c(C)noc2C)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BromoELECT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02232
BindingDB Entry DOI: 10.7270/Q2NS0ZQF |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50571557

(CHEMBL4853072)Show SMILES COc1ccc(CCc2nc3cc(ccn3c2NC2CCCCC2)-c2c(C)noc2C)cc1Cl | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BromoELECT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02232
BindingDB Entry DOI: 10.7270/Q2NS0ZQF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50571569

(CHEMBL4875989)Show SMILES [H][C@]12CS[C@@H](CCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCC(=O)N3CCN(CC3)c3ccc(cc3)-c3nc4ccc(cn4c3NC3CCCCC3)-c3c(C)noc3C)[C@@]1([H])NC(=O)N2 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BromoELECT assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02232
BindingDB Entry DOI: 10.7270/Q2NS0ZQF |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579718

(CHEMBL5083683)Show SMILES COc1ccc2CCCN(CCC\C=C\c3cccc4NC(=O)C[C@H](C)Nc34)c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human EP300 assessed as dissociation constant by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00348
BindingDB Entry DOI: 10.7270/Q2RF5ZW2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579715
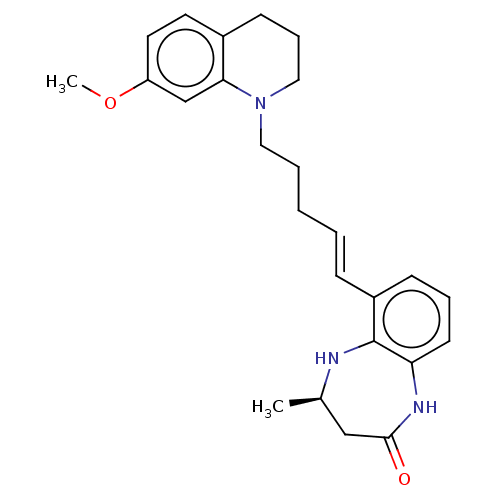
(CHEMBL5089448)Show SMILES COc1ccc2CCCN(CCC\C=C\c3cccc4NC(=O)C[C@@H](C)Nc34)c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human EP300 assessed as dissociation constant by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00348
BindingDB Entry DOI: 10.7270/Q2RF5ZW2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50584925
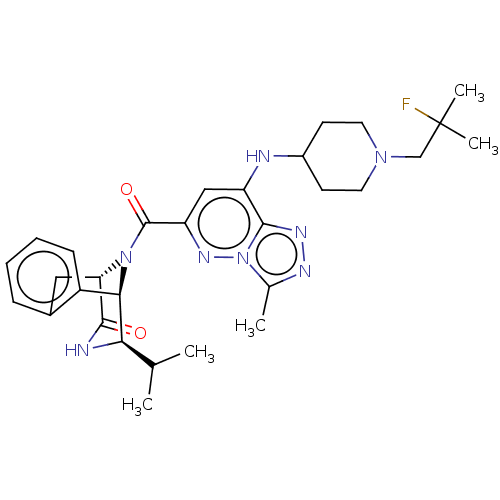
(CHEMBL5079885)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(C)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:3.8.2:13.12.11| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | <3.16E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human EP300 bromodomain assessed as dissociation constant by BROMOscan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50603097
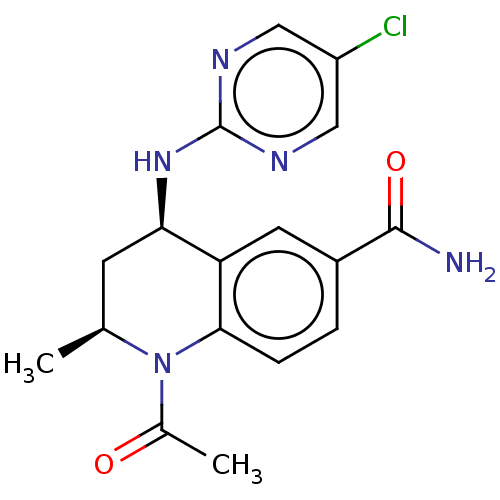
(CHEMBL5195288)Show SMILES C[C@H]1C[C@@H](Nc2ncc(Cl)cn2)c2cc(ccc2N1C(C)=O)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01747
BindingDB Entry DOI: 10.7270/Q23B646M |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM449844
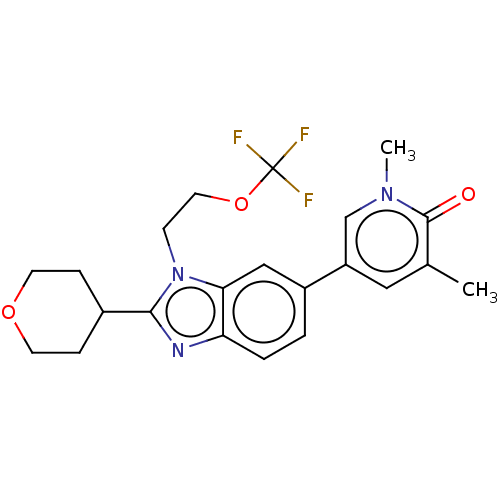
(1,3-dimethyl-5-[2-tetrahydropyran-4-yl-3-[2-(trifl...)Show SMILES Cc1cc(cn(C)c1=O)-c1ccc2nc(C3CCOCC3)n(CCOC(F)(F)F)c2c1 Show InChI InChI=1S/C22H24F3N3O3/c1-14-11-17(13-27(2)21(14)29)16-3-4-18-19(12-16)28(7-10-31-22(23,24)25)20(26-18)15-5-8-30-9-6-15/h3-4,11-13,15H,5-10H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | CHEMBL5282240
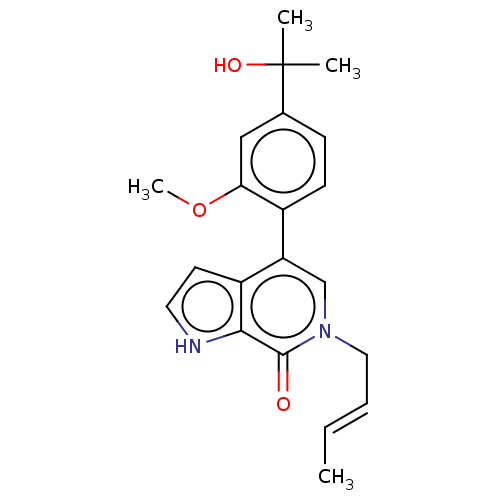
| PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM321437
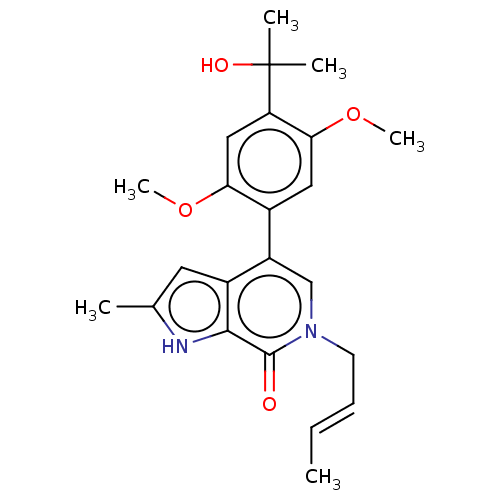
((E)-6-(but-2-en-1-yl)-4-(4-(2-hydroxypropan-2-yl)-...)Show SMILES COc1cc(c(OC)cc1-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12)C(C)(C)O Show InChI InChI=1S/C23H28N2O4/c1-7-8-9-25-13-17(16-10-14(2)24-21(16)22(25)26)15-11-20(29-6)18(23(3,4)27)12-19(15)28-5/h7-8,10-13,24,27H,9H2,1-6H3/b8-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data