 Found 11 hits of ki for UniProtKB: Q09472
Found 11 hits of ki for UniProtKB: Q09472 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50456444

(CHEMBL3734823)Show SMILES CC1=NN(C(=O)\C1=C\c1ccc(o1)-c1cc(C)c(C)cc1[N+]([O-])=O)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of VMA intein chitin binding domain-fused p300 HAT domain (1287 to 1652 residues) (unknown origin) expressed in Escherichia coli BL21(RIL)... |
Eur J Med Chem 138: 320-327 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.037
BindingDB Entry DOI: 10.7270/Q2ZW1PHN |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of synthetic VMA-tagged p300 (1287 to 1652 residues) (unknown origin) expressed in Escherichia coli BL21(RIL)-DE3 cells using H4-15 peptid... |
J Med Chem 59: 1249-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01502
BindingDB Entry DOI: 10.7270/Q2DV1MRN |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Competitive inhibition of p300 HAT |
Bioorg Med Chem 19: 3605-15 (2011)
Article DOI: 10.1016/j.bmc.2011.01.029
BindingDB Entry DOI: 10.7270/Q2SN0992 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112915
BindingDB Entry DOI: 10.7270/Q2CV4NQF |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50453820

(CHEMBL4208500)Show SMILES CC1=NN(C(=O)C1=Cc1ccc(o1)-c1cc(C)c(C)cc1[N+]([O-])=O)c1ccc(cc1)C(O)=O |t:1| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of VMA intein chitin binding domain-fused p300 HAT domain M1652G mutant (unknown origin) expressed in Escherichia coli BL21(RI... |
J Med Chem 61: 3239-3252 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01817
BindingDB Entry DOI: 10.7270/Q2542R5N |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081125
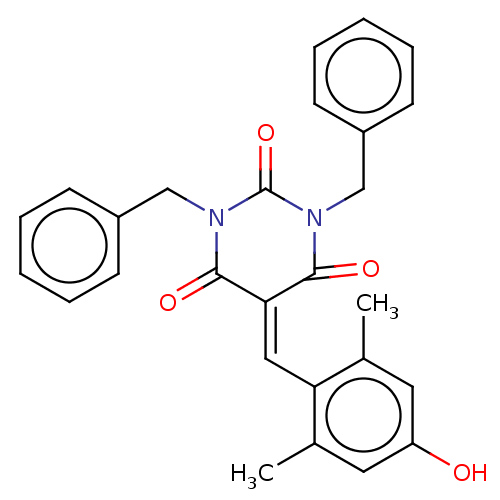
(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (unknown origin) using acetyl CoA as substrate after 15 mins by double reciprocal plot analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50377962
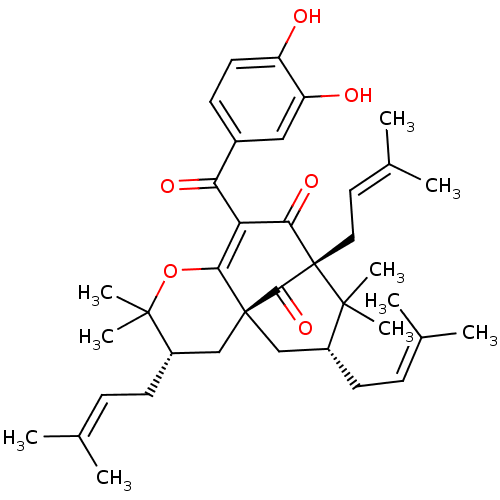
(CAMBOGIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6@H]1-[#6][C@@]23[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c4ccc(-[#8])c(-[#8])c4)=[#6]2-[#8]C1([#6])[#6])[#6]3=O |r,c:37,TLB:43:42:15.8.9:26.24.37| Show InChI InChI=1S/C38H50O6/c1-22(2)11-14-26-20-37-21-27(15-12-23(3)4)36(9,10)44-33(37)30(31(41)25-13-16-28(39)29(40)19-25)32(42)38(34(37)43,35(26,7)8)18-17-24(5)6/h11-13,16-17,19,26-27,39-40H,14-15,18,20-21H2,1-10H3/t26-,27+,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Inhibition of HAT p300 catalytic domain by equilibrium dialysis |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50241990

(CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@@H](-[#6][C@@]12[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6]1=O)[#6]2=O)-[#6](-[#6])=[#6] |r,TLB:40:39:26.24.37:15.8.9| Show InChI InChI=1S/C38H50O6/c1-22(2)11-13-27(25(7)8)20-37-21-28(15-12-23(3)4)36(9,10)38(35(37)44,18-17-24(5)6)34(43)31(33(37)42)32(41)26-14-16-29(39)30(40)19-26/h11-12,14,16-17,19,27-28,31,39-40H,7,13,15,18,20-21H2,1-6,8-10H3/t27-,28+,31?,37+,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Inhibition of HAT p300 catalytic domain by equilibrium dialysis |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50265449

((1R,3S,9S,11R)-7-(3-Hydroxy-4-methoxy-benzoyl)-4,4...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2)[#6]-1=O |r,c:12| Show InChI InChI=1S/C39H54O5/c1-24(2)12-15-28-21-38-22-29(16-13-25(3)4)37(9,10)44-35(38)32(33(41)27-14-17-31(43-11)30(40)20-27)34(42)39(23-38,36(28,7)8)19-18-26(5)6/h12-14,17-18,20,28-29,40H,15-16,19,21-23H2,1-11H3/t28-,29+,38-,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru Centre for Advanced Scientific Research
Curated by ChEMBL
| Assay Description
Inhibition of HAT p300 catalytic domain by equilibrium dialysis |
J Med Chem 52: 267-77 (2009)
Article DOI: 10.1021/jm800657z
BindingDB Entry DOI: 10.7270/Q2445NDC |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50081125

(CHEMBL3421961)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1\[#6]=[#6]-1/[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6](=O)-[#7](-[#6]-c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C27H24N2O4/c1-18-13-22(30)14-19(2)23(18)15-24-25(31)28(16-20-9-5-3-6-10-20)27(33)29(26(24)32)17-21-11-7-4-8-12-21/h3-15,30H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of P300 (unknown origin) using biotinylated H3 as substrate after 15 mins by double reciprocal plot analysis |
J Med Chem 58: 2779-98 (2015)
Article DOI: 10.1021/jm5019687
BindingDB Entry DOI: 10.7270/Q2736SMP |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50260093
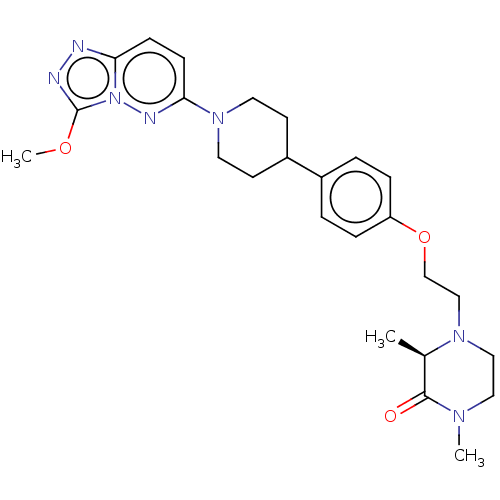
(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human EP300 expressed in bacterial expression system |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data