

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM152497 ((E)-N'-(6-Bromo-2-oxoindolin-3-ylidene)-2-(2,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hazara University | Assay Description 10 無 of test samples (5 mg/mL DMSO solution) were reconstituted in 100 無 of 100 mM-phosphate buffer (pH6.8) in 96-well microplate and incubated wit... | Bioorg Chem 60: 42-8 (2015) Article DOI: 10.1016/j.bioorg.2015.03.005 BindingDB Entry DOI: 10.7270/Q2DR2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM152499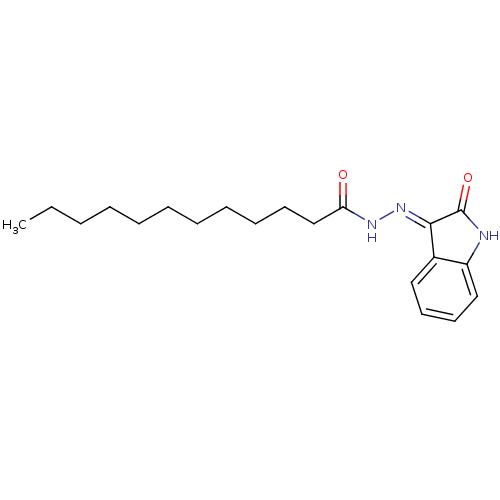 ((E)-N'-(2-oxoindolin-3-ylidene)dodecanehydrazi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hazara University | Assay Description 10 無 of test samples (5 mg/mL DMSO solution) were reconstituted in 100 無 of 100 mM-phosphate buffer (pH6.8) in 96-well microplate and incubated wit... | Bioorg Chem 60: 42-8 (2015) Article DOI: 10.1016/j.bioorg.2015.03.005 BindingDB Entry DOI: 10.7270/Q2DR2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM152496 ((E)-N'-(6-bromo-2-oxoindolin-3-ylidene)-2-(3,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hazara University | Assay Description 10 無 of test samples (5 mg/mL DMSO solution) were reconstituted in 100 無 of 100 mM-phosphate buffer (pH6.8) in 96-well microplate and incubated wit... | Bioorg Chem 60: 42-8 (2015) Article DOI: 10.1016/j.bioorg.2015.03.005 BindingDB Entry DOI: 10.7270/Q2DR2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308256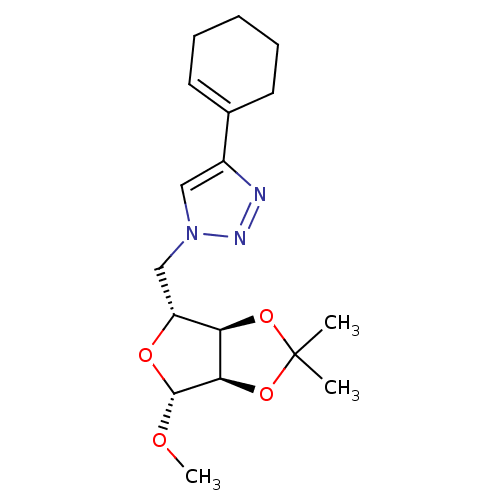 (1-O-Methyl-2,3-O-isopropylidene-5-(4-cyclohexene-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308259 (1-O-Methyl-2,3-O-isopropylidene-5-[4-(1-hydroxycyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308258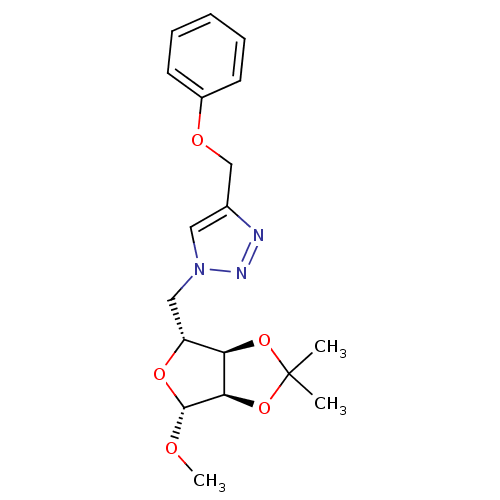 (1-O-Methyl-2,3-O-isopropylidene-5-(4-phenoxymethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181062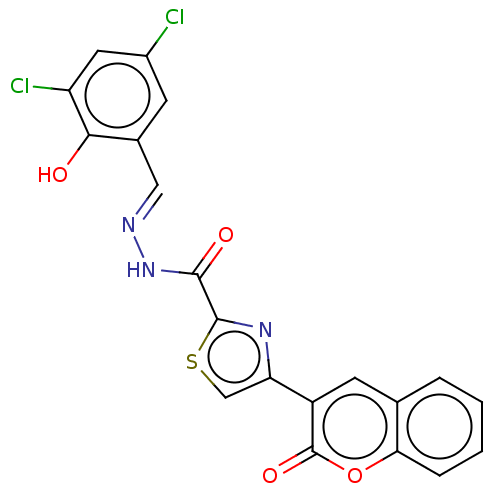 ((E)-N'-(3,5-Dichloro-2-hydroxybenzylidene)-4-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181064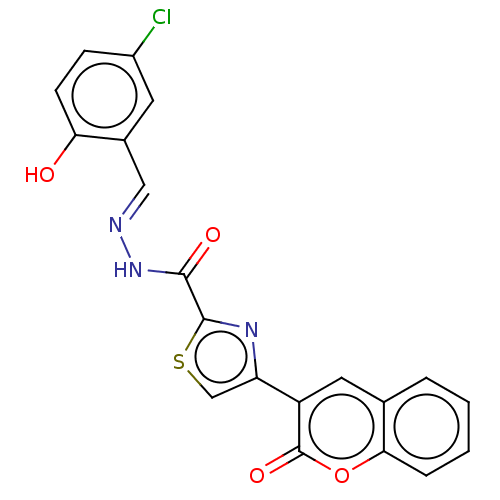 ((E)-N'-(5-Chloro-2-hydroxybenzylidene)-4-(2-ox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.23E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84968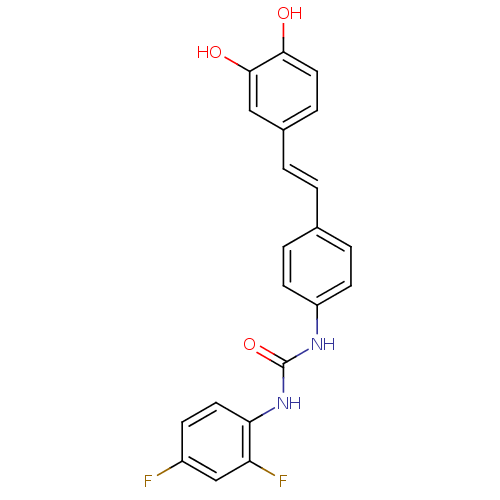 (Urea derivative, 12) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM243075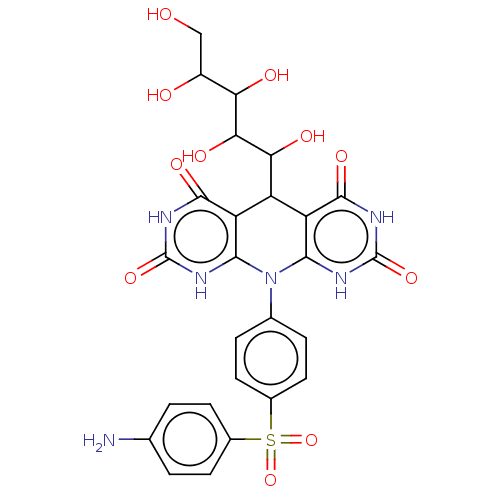 (α-Gl inhibitor, C3) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shiraz University | Assay Description In this study, the inhibition assay of yeast enzyme was performed in 100 mM phosphate buffer pH 7.0 at 25蚓 with minor changes, according to the meth... | J Enzyme Inhib Med Chem 28: 1228-35 (2013) Article DOI: 10.3109/14756366.2012.727812 BindingDB Entry DOI: 10.7270/Q2XP73V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181061 ((E)-N'-(3,5-Di-tert-butyl-2-hydroxybenzylidene...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181068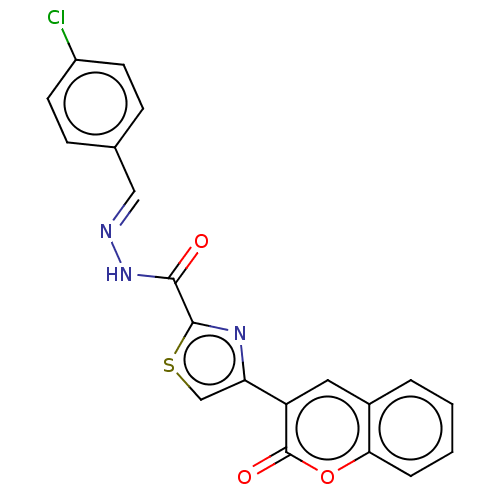 ((E)-N'-(4-Chlorobenzylidene)-4-(2-oxo-2H-chrom...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM152498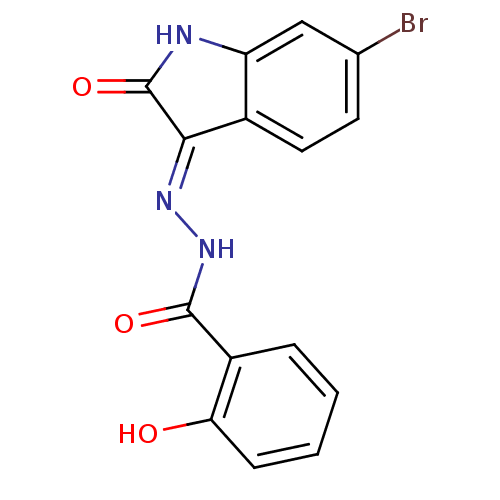 ((E)-N'-(6-bromo-2-oxoindolin-3-ylidene)-2- hyd...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hazara University | Assay Description 10 無 of test samples (5 mg/mL DMSO solution) were reconstituted in 100 無 of 100 mM-phosphate buffer (pH6.8) in 96-well microplate and incubated wit... | Bioorg Chem 60: 42-8 (2015) Article DOI: 10.1016/j.bioorg.2015.03.005 BindingDB Entry DOI: 10.7270/Q2DR2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181069 ((E)-N'-(3-Chlorobenzylidene)-4-(2-oxo-2H-chrom...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181073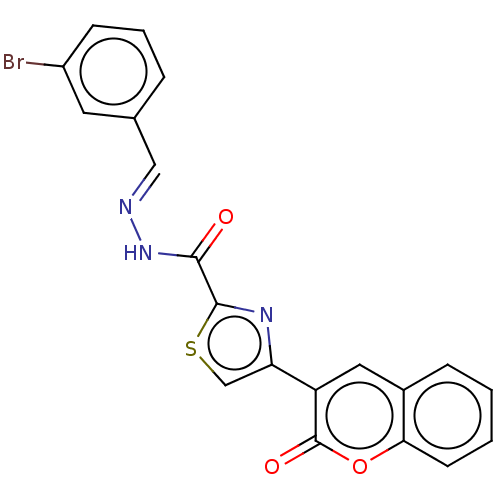 ((E)-N'-(3-bromobenzylidene)-4-(2-oxo-2H-chrome...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84969 (Urea derivative, 13) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308255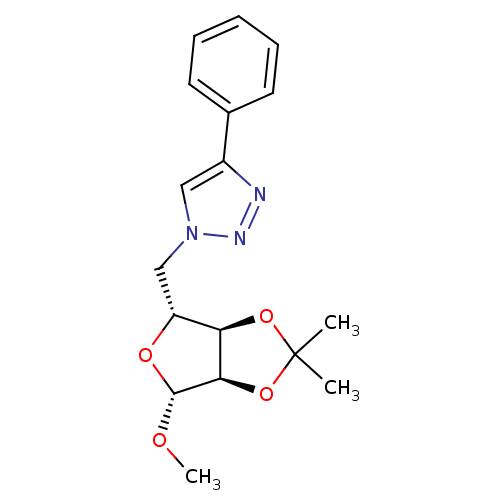 (1-O-Methyl-2,3-O-isopropylidene-5-(4-phenyl-1H-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308257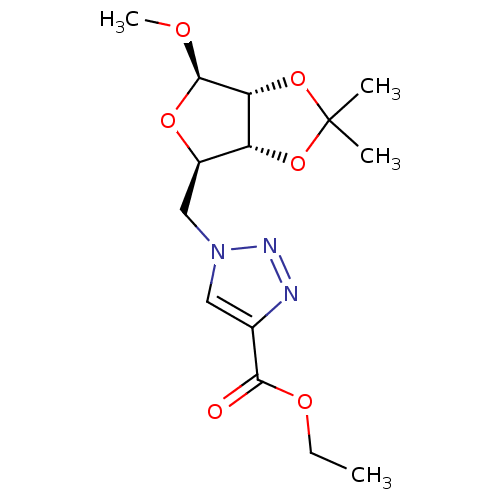 (1-O-Methyl-2,3-O-isopropylidene-5-(4-carboxylate e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181070 ((E)-4-(2-Oxo-2H-chromen-3-yl)-N'-(4-(trifluoro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181063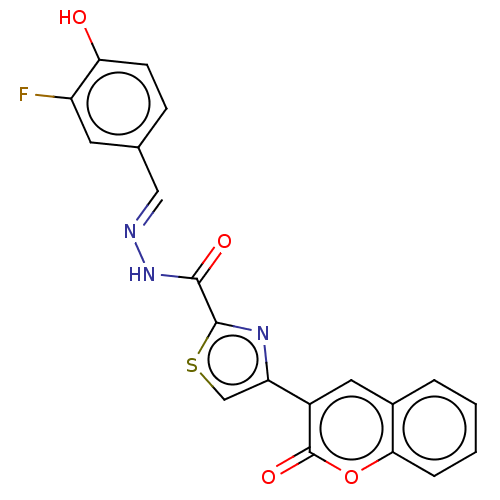 ((E)-N'-(3-Fluoro-4-hydroxybenzylidene)-4-(2-ox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181067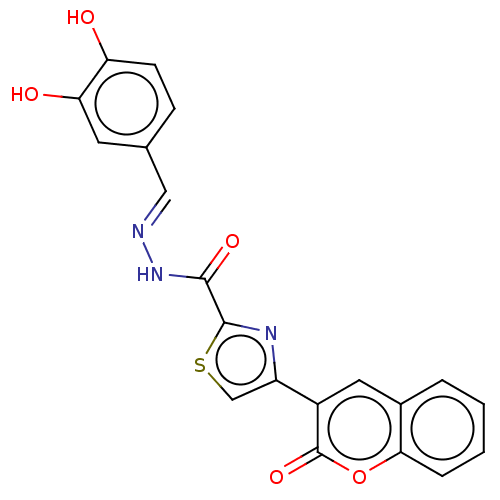 ((E)-N'-(3,4-Dihydroxybenzylidene)-4-(2-oxo-2H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM152494 ((E)-2-(2,4-dichlorophenyl)-N'-(2-oxo-1-propyli...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hazara University | Assay Description 10 無 of test samples (5 mg/mL DMSO solution) were reconstituted in 100 無 of 100 mM-phosphate buffer (pH6.8) in 96-well microplate and incubated wit... | Bioorg Chem 60: 42-8 (2015) Article DOI: 10.1016/j.bioorg.2015.03.005 BindingDB Entry DOI: 10.7270/Q2DR2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84972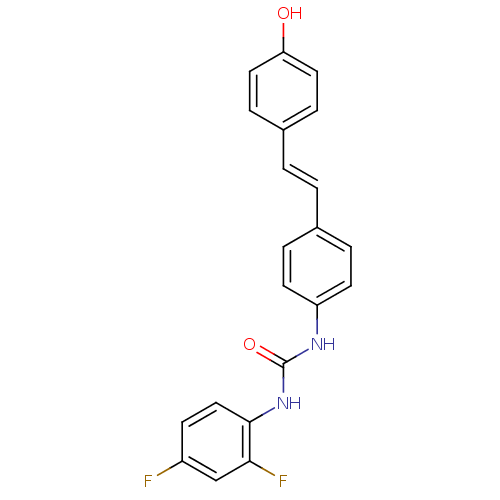 (Urea derivative, 16) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84963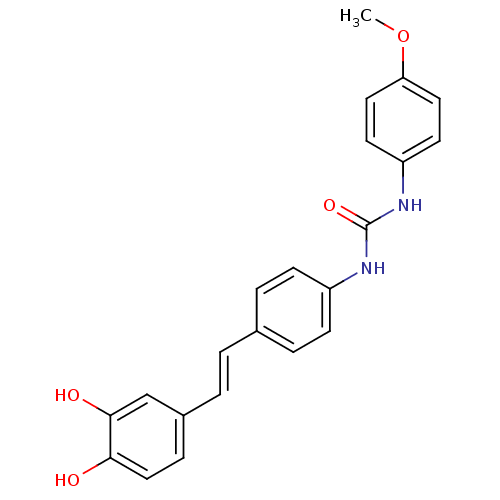 (Urea derivative, 7) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+4 | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84967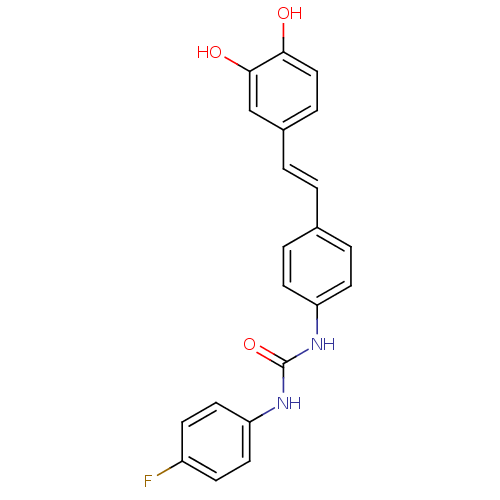 (Urea derivative, 11) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20E+3 | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM60591 ((E)-N'-(3-Fluorobenzylidene)-4-(2-oxo-2H-chromen-3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181071 ((E)-N'-(2-Fluorobenzylidene)-4-(2-oxo-2H-chrom...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM243068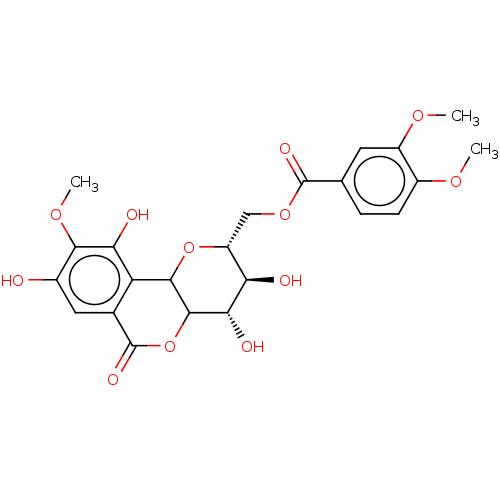 (11-O-(3',4'-dimethoxybenzoyl)-bergenin (6)) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Kinki University | Assay Description α-glucosidase (25 μL, 0.2 U/mL), 25 μL of various concentrations of samples, and 175 μL of 50 mM sodium phosphate buffer (pH 7.0)... | J Enzyme Inhib Med Chem 28: 1162-70 (2013) Article DOI: 10.3109/14756366.2012.719503 BindingDB Entry DOI: 10.7270/Q22F7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308260 (1-O-Methyl-2,3-O-isopropylidene-5-[4-(tetrahydro-2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM243074 (α-Gl inhibitor, C2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Shiraz University | Assay Description In this study, the inhibition assay of yeast enzyme was performed in 100 mM phosphate buffer pH 7.0 at 25蚓 with minor changes, according to the meth... | J Enzyme Inhib Med Chem 28: 1228-35 (2013) Article DOI: 10.3109/14756366.2012.727812 BindingDB Entry DOI: 10.7270/Q2XP73V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181060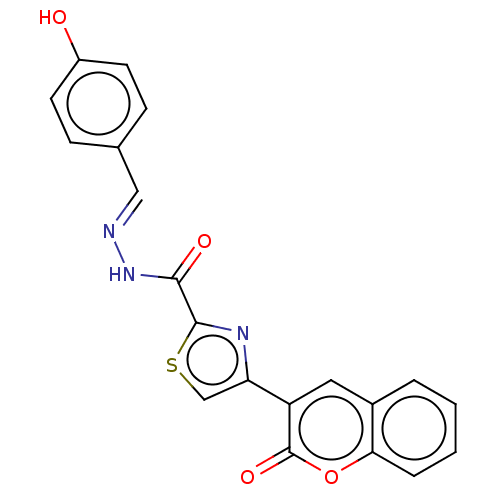 ((E)-N'-(4-Hydroxybenzylidene)-4-(2-oxo-2H-chro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.15E+4 | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84966 (Urea derivative, 10) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84970 (Urea derivative, 14) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181065 ((E)-N'-(2-Hydroxy-4-methoxybenzylidene)-4-(2-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50180587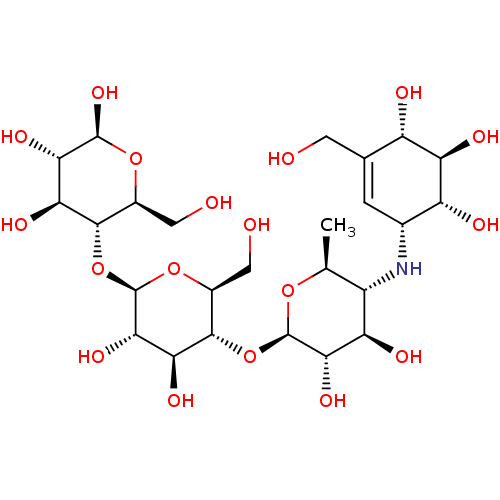 (CHEMBL3734896) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Technology Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 using 4-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 30 mins followed by substrate a... | Bioorg Med Chem Lett 26: 4007-14 (2016) Article DOI: 10.1016/j.bmcl.2016.06.086 BindingDB Entry DOI: 10.7270/Q22B910H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50207546 ((-)-epivaliolamine | CHEMBL223096) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University Curated by ChEMBL | Assay Description Inhibition of baker's yeast alpha-glucosidase | J Nat Prod 70: 493-7 (2007) Article DOI: 10.1021/np068069t BindingDB Entry DOI: 10.7270/Q28S4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181059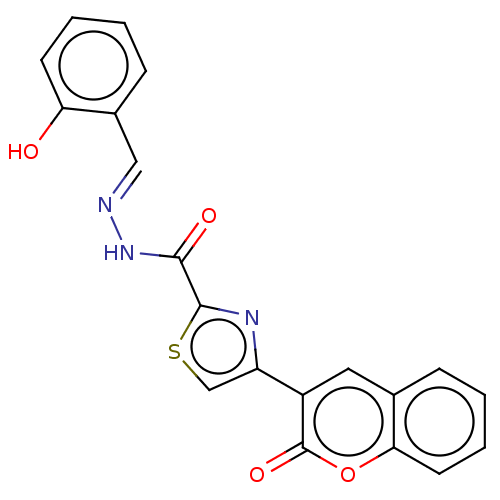 ((E)-N'-(2-Hydroxybenzylidene)-4-(2-oxo-2H-chro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50333465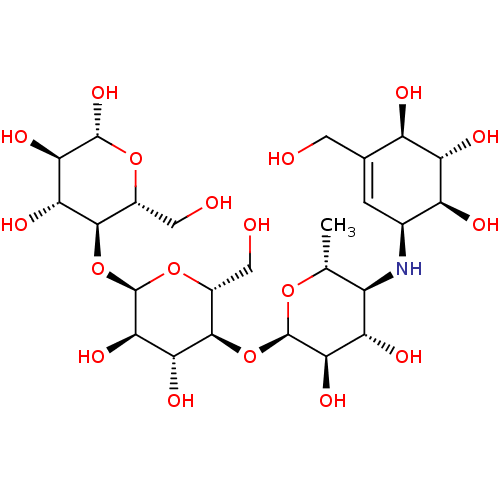 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | 6.8 | 37 |
COMSATS Institute of Information Technology, Lahore 54000, Pakistan | Assay Description Reaction mixture (100 µL) contained 70 µL 50 mM Na2HPO4 buffer, pH 6.8, 10 µL test compound (0.5 mM), followed by the addition of 10 &... | Bioorg Chem 54: 96-104 (2014) Article DOI: 10.1016/j.bioorg.2014.05.003 BindingDB Entry DOI: 10.7270/Q2NG4P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181066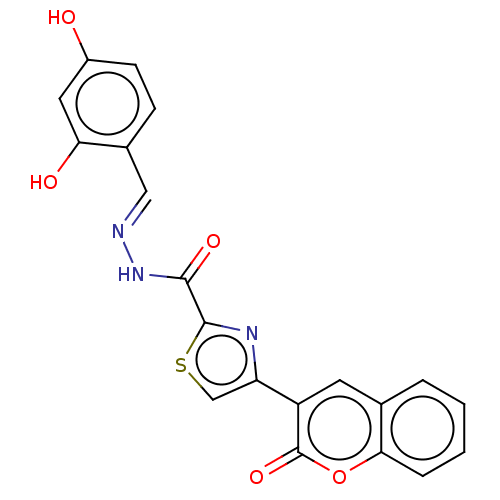 ((E)-N'-(2,4-Dihydroxybenzylidene)-4-(2-oxo-2H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84962 (Urea derivative, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+4 | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM23406 ((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.33E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84959 (Stilbene derivative, 2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.51E+4 | n/a | 4.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50190320 (CHEMBL3827545) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Technology Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 using 4-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 30 mins followed by substrate a... | Bioorg Med Chem Lett 26: 4007-14 (2016) Article DOI: 10.1016/j.bmcl.2016.06.086 BindingDB Entry DOI: 10.7270/Q22B910H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84973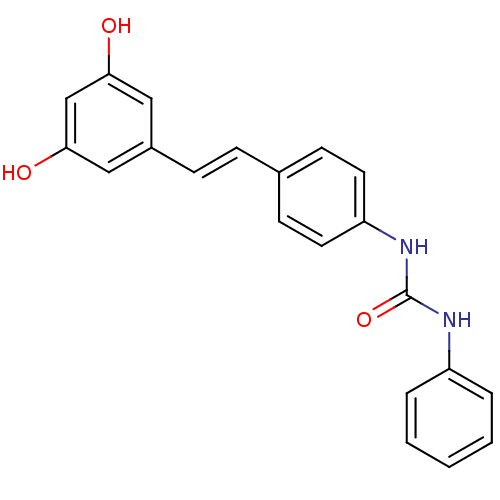 (Urea derivative, 17) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84971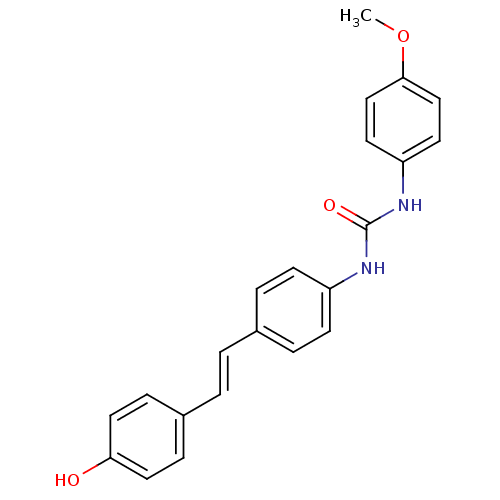 (Urea derivative, 15) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84965 (Urea derivative, 9) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM181074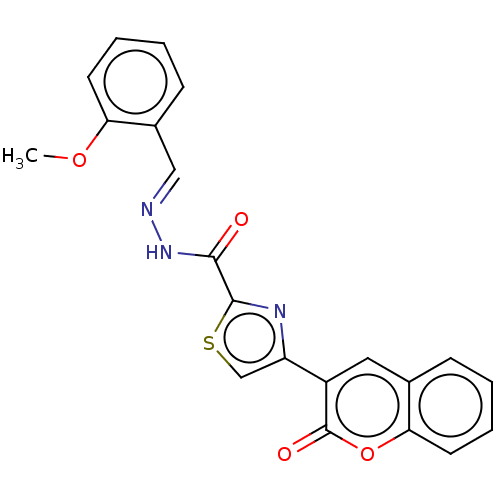 ((E)-N'-(2-Methoxybenzylidene)-4-(2-oxo-2H-chro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Jishou University | Assay Description The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u... | Bioorg Chem 65: 167-74 (2016) Article DOI: 10.1016/j.bioorg.2016.03.001 BindingDB Entry DOI: 10.7270/Q2445K8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM84975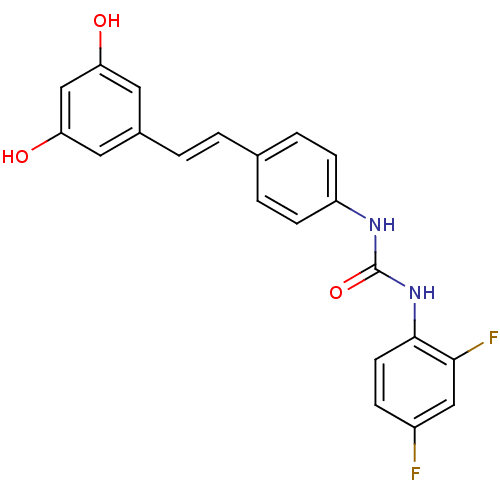 (Urea derivative, 19) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University | Assay Description All enzymatic activities were determined by using the appropriate substrate (p-nitrophenyl-alpha-D-glucopyranoside, p-nitrophenyl-beta-D-gulcopyranos... | Chembiochem 11: 2125-31 (2010) Article DOI: 10.1002/cbic.201000376 BindingDB Entry DOI: 10.7270/Q2542M33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 104 total ) | Next | Last >> |