

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50225280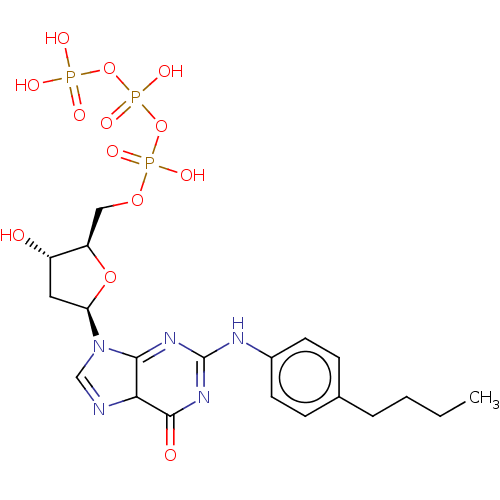 (CHEMBL3144215) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50293303 (CHEMBL498103 | gallic acid 5,6-dihydroxy-3-carboxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DNA polymerase alpha from human KB3 cells | J Nat Prod 53: 1234-1240 (1990) Article DOI: 10.1021/np50071a015 BindingDB Entry DOI: 10.7270/Q27P8ZC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025592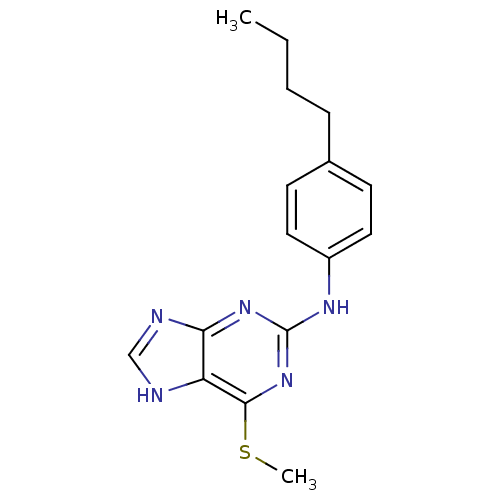 ((4-Butyl-phenyl)-(6-methylsulfanyl-9H-purin-2-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025600 ((4-Butyl-phenyl)-(6-methoxy-9H-purin-2-yl)-amine |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025598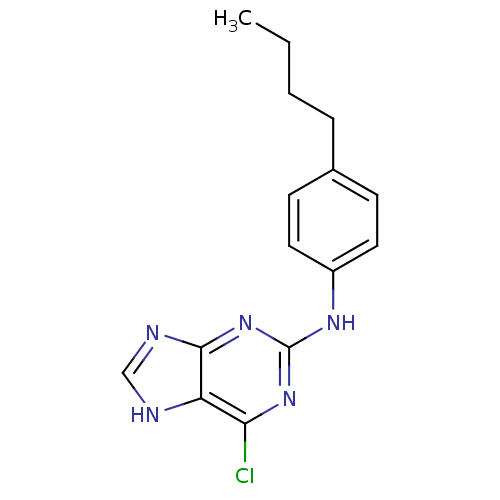 ((4-Butyl-phenyl)-(6-chloro-9H-purin-2-yl)-amine | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106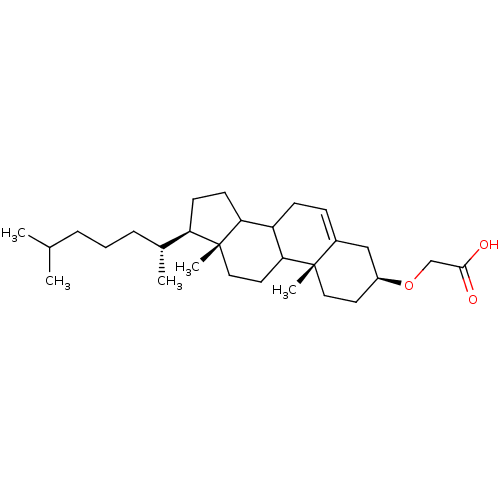 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025597 ((4-Butyl-phenyl)-(9H-purin-2-yl)-amine | CHEMBL278...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013074 (2-(4-Butyl-phenylamino)-1,9-dihydro-purin-6-one | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111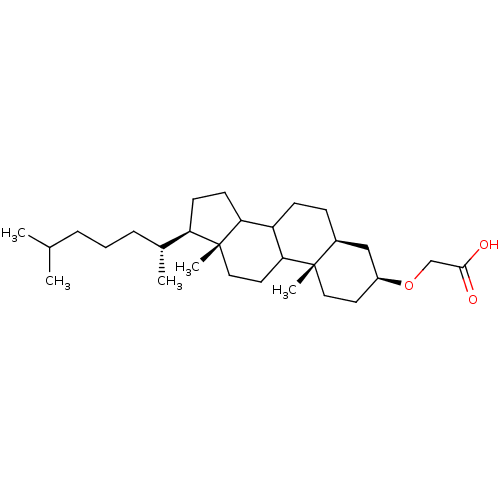 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153113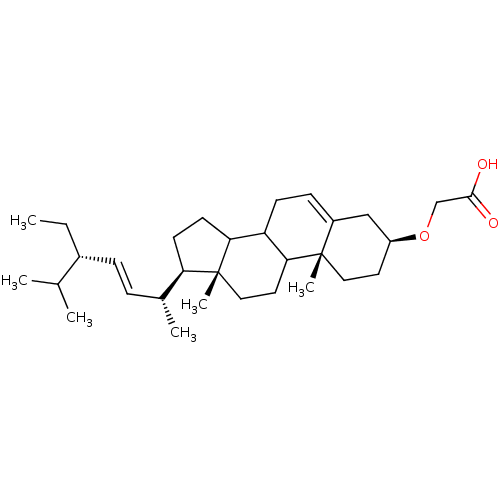 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013074 (2-(4-Butyl-phenylamino)-1,9-dihydro-purin-6-one | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025593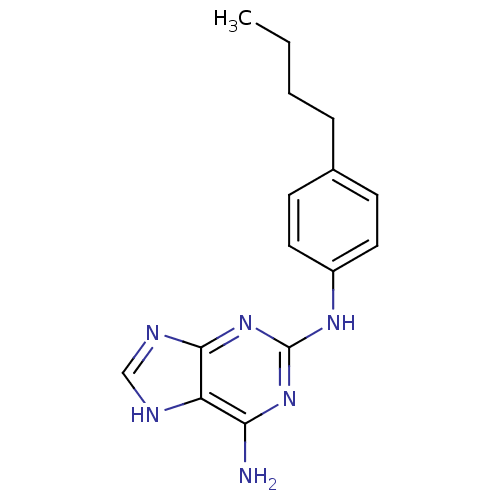 (CHEMBL16122 | N*2*-(4-Butyl-phenyl)-9H-purine-2,6-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50405103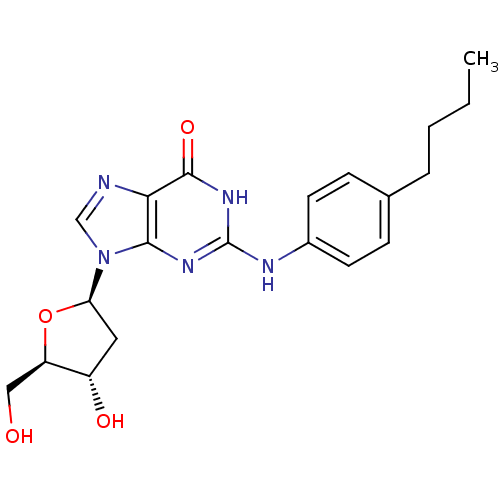 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153113 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50025595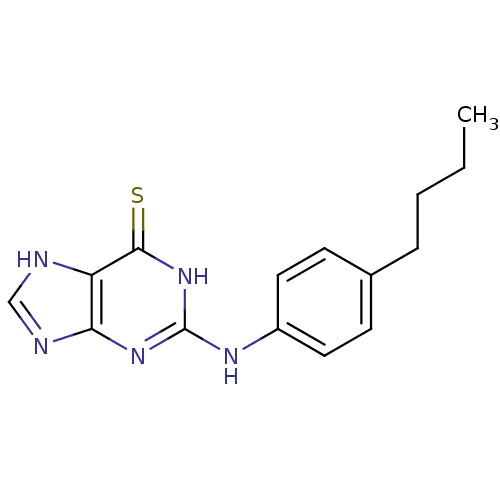 (2-(4-Butyl-phenylamino)-1,9-dihydro-purine-6-thion...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50405103 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50405103 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013075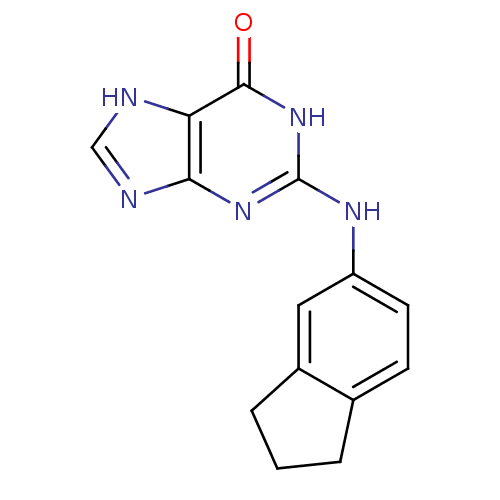 (2-(Indan-5-ylamino)-1,9-dihydro-purin-6-one | CHEM...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50118823 (2,2-Dimethyl-propionic acid [5-(7-amino-[1,2,3]tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the incorporation of dATP into DNA by DNA polymerase alpha | J Med Chem 45: 4505-12 (2002) BindingDB Entry DOI: 10.7270/Q2KS6QWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50118822 (2,2-Dimethyl-propionic acid [5-(6-amino-8-bromo-pu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the incorporation of dATP into DNA by DNA polymerase alpha | J Med Chem 45: 4505-12 (2002) BindingDB Entry DOI: 10.7270/Q2KS6QWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50013093 (2-(3-Ethyl-4-methyl-phenylamino)-1,9-dihydro-purin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.76E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the concentration which gives half-maximal inhibition of Chinese Hamster Ovary DNA polymerase alpha | J Med Chem 30: 109-16 (1987) BindingDB Entry DOI: 10.7270/Q2ZG6R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||