 Found 132 hits of ki for UniProtKB: P10276
Found 132 hits of ki for UniProtKB: P10276 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards human Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2313-2318 (1997)
Article DOI: 10.1016/S0960-894X(97)00420-4
BindingDB Entry DOI: 10.7270/Q2Z89CDD |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178953
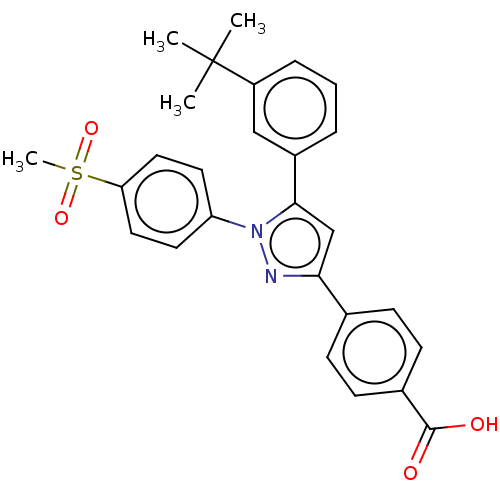
(CHEMBL3815166)Show SMILES CC(C)(C)c1cccc(c1)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C27H26N2O4S/c1-27(2,3)21-7-5-6-20(16-21)25-17-24(18-8-10-19(11-9-18)26(30)31)28-29(25)22-12-14-23(15-13-22)34(4,32)33/h5-17H,1-4H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178952

(CHEMBL3814574)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H32N2O4S/c1-30(2)16-17-31(3,4)26-18-22(10-15-25(26)30)28-19-27(20-6-8-21(9-7-20)29(34)35)32-33(28)23-11-13-24(14-12-23)38(5,36)37/h6-15,18-19H,16-17H2,1-5H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290137
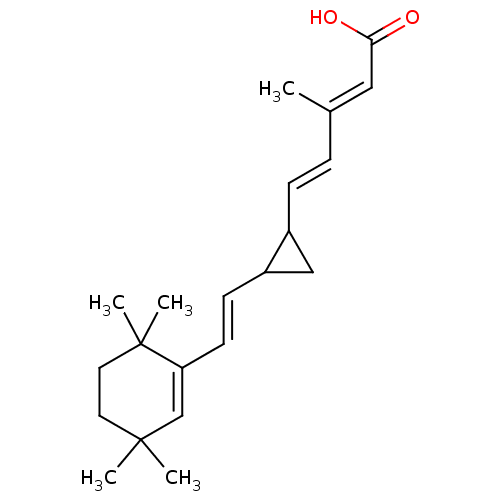
((2E,4E)-3-Methyl-5-{2-[(E)-2-(3,3,6,6-tetramethyl-...)Show SMILES C\C(\C=C\C1CC1\C=C\C1=CC(C)(C)CCC1(C)C)=C/C(O)=O |t:10| Show InChI InChI=1S/C21H30O2/c1-15(12-19(22)23)6-7-16-13-17(16)8-9-18-14-20(2,3)10-11-21(18,4)5/h6-9,12,14,16-17H,10-11,13H2,1-5H3,(H,22,23)/b7-6+,9-8+,15-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards human Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2313-2318 (1997)
Article DOI: 10.1016/S0960-894X(97)00420-4
BindingDB Entry DOI: 10.7270/Q2Z89CDD |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048296
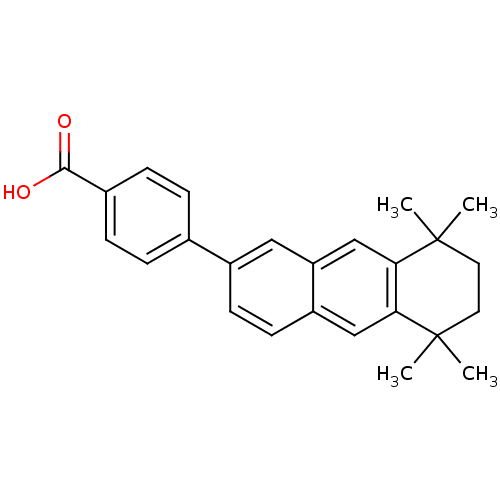
(4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-anthrace...)Show SMILES CC1(C)CCC(C)(C)c2cc3cc(ccc3cc12)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H26O2/c1-24(2)11-12-25(3,4)22-15-20-13-18(9-10-19(20)14-21(22)24)16-5-7-17(8-6-16)23(26)27/h5-10,13-15H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178954

(CHEMBL3813779)Show SMILES Cc1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C28H28N2O4S/c1-18-14-21(16-22(15-18)28(2,3)4)26-17-25(19-6-8-20(9-7-19)27(31)32)29-30(26)23-10-12-24(13-11-23)35(5,33)34/h6-17H,1-5H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625
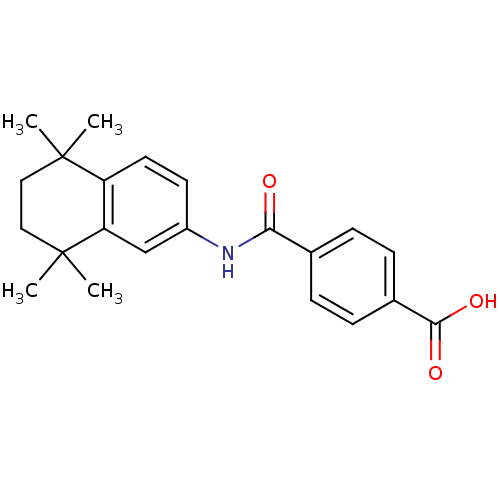
(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178961
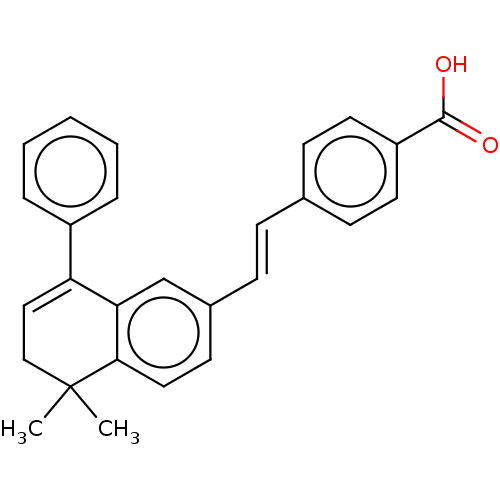
(CHEMBL2385268)Show SMILES CC1(C)CC=C(c2ccccc2)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 |t:4| Show InChI InChI=1S/C27H24O2/c1-27(2)17-16-23(21-6-4-3-5-7-21)24-18-20(12-15-25(24)27)9-8-19-10-13-22(14-11-19)26(28)29/h3-16,18H,17H2,1-2H3,(H,28,29)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219
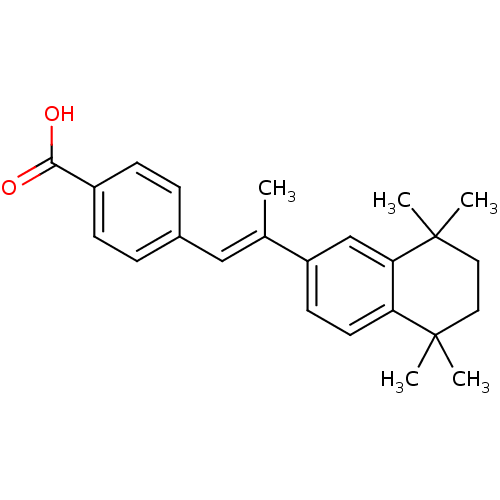
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to Retinoic acid receptor RAR alpha |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50129720
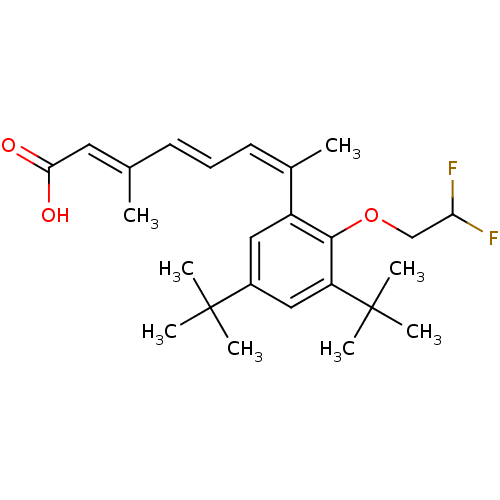
((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34F2O3/c1-16(12-22(28)29)10-9-11-17(2)19-13-18(24(3,4)5)14-20(25(6,7)8)23(19)30-15-21(26)27/h9-14,21H,15H2,1-8H3,(H,28,29)/b10-9+,16-12+,17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Binding affinity against RAR alpha receptor using [3H]-ATRA as radioligand in CV-1 cells |
Bioorg Med Chem Lett 13: 4071-5 (2003)
BindingDB Entry DOI: 10.7270/Q2V987GP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052033
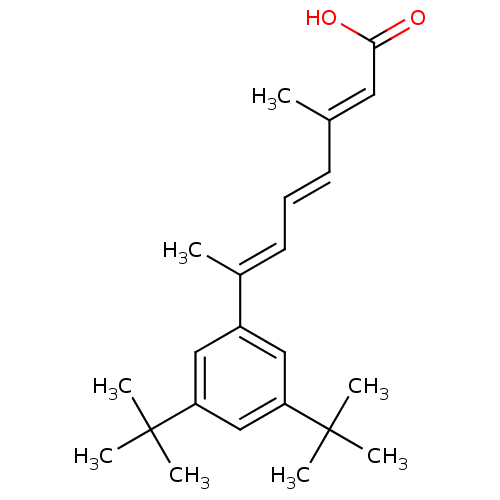
((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to Retinoic acid receptor RAR alpha |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052033

((2E,4E,6E)-7-(3,5-Di-tert-butyl-phenyl)-3-methyl-o...)Show SMILES C\C(\C=C\C=C(/C)c1cc(cc(c1)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C23H32O2/c1-16(12-21(24)25)10-9-11-17(2)18-13-19(22(3,4)5)15-20(14-18)23(6,7)8/h9-15H,1-8H3,(H,24,25)/b10-9+,16-12+,17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to Retinoic acid receptor RAR alpha |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178955

(CHEMBL3813965)Show SMILES CC(C)c1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C30H32N2O4S/c1-19(2)22-15-23(17-24(16-22)30(3,4)5)28-18-27(20-7-9-21(10-8-20)29(33)34)31-32(28)25-11-13-26(14-12-25)37(6,35)36/h7-19H,1-6H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143824
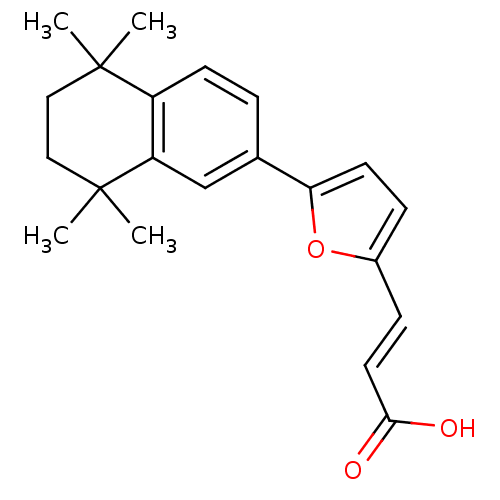
((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc(\C=C\C(O)=O)o1 Show InChI InChI=1S/C21H24O3/c1-20(2)11-12-21(3,4)17-13-14(5-8-16(17)20)18-9-6-15(24-18)7-10-19(22)23/h5-10,13H,11-12H2,1-4H3,(H,22,23)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048295
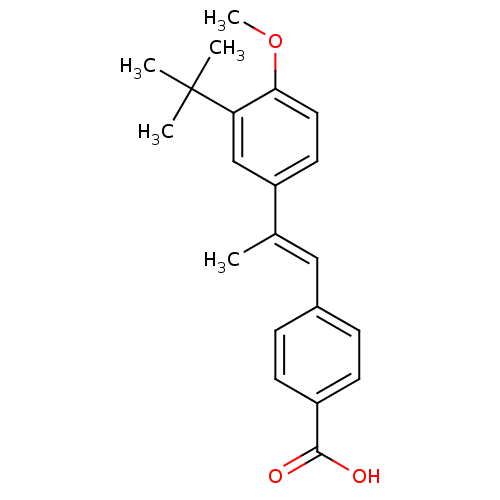
(4-[(E)-2-(3-tert-Butyl-4-methoxy-phenyl)-propenyl]...)Show SMILES COc1ccc(cc1C(C)(C)C)C(\C)=C\c1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H24O3/c1-14(12-15-6-8-16(9-7-15)20(22)23)17-10-11-19(24-5)18(13-17)21(2,3)4/h6-13H,1-5H3,(H,22,23)/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to Retinoic acid receptor RAR alpha |
J Med Chem 39: 2659-63 (1996)
Article DOI: 10.1021/jm960285j
BindingDB Entry DOI: 10.7270/Q2988631 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50052588
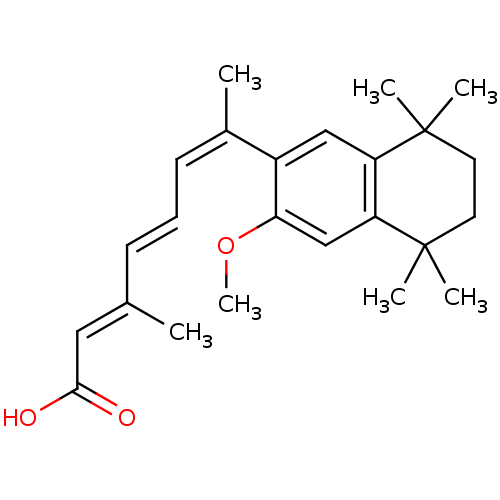
((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...)Show SMILES COc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H32O3/c1-16(13-22(25)26)9-8-10-17(2)18-14-19-20(15-21(18)27-7)24(5,6)12-11-23(19,3)4/h8-10,13-15H,11-12H2,1-7H3,(H,25,26)/b9-8+,16-13+,17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to Retinoic acid receptor RAR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048285
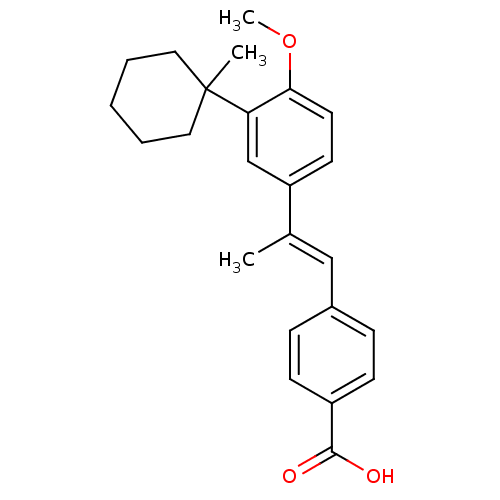
(4-{(E)-2-[4-Methoxy-3-(1-methyl-cyclohexyl)-phenyl...)Show SMILES COc1ccc(cc1C1(C)CCCCC1)C(\C)=C\c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H28O3/c1-17(15-18-7-9-19(10-8-18)23(25)26)20-11-12-22(27-3)21(16-20)24(2)13-5-4-6-14-24/h7-12,15-16H,4-6,13-14H2,1-3H3,(H,25,26)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048277
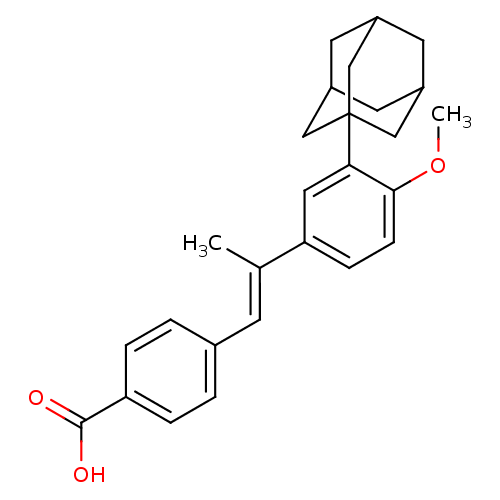
(4-[(E)-2-(3-Adamantan-1-yl-4-methoxy-phenyl)-prope...)Show SMILES COc1ccc(cc1C12CC3CC(CC(C3)C1)C2)C(\C)=C\c1ccc(cc1)C(O)=O |TLB:15:14:17:9.10.11,15:10:17:16.14.13,THB:13:14:9:17.12.11,13:12:9:16.14.15| Show InChI InChI=1S/C27H30O3/c1-17(9-18-3-5-22(6-4-18)26(28)29)23-7-8-25(30-2)24(13-23)27-14-19-10-20(15-27)12-21(11-19)16-27/h3-9,13,19-21H,10-12,14-16H2,1-2H3,(H,28,29)/b17-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/alpha
(Homo sapiens (Human)) | BDBM50178956

(CHEMBL3813807)Show SMILES CC(C)(C)c1cc(cc(c1)C(C)(C)C)-c1cc(nn1-c1ccc(cc1)S(C)(=O)=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H34N2O4S/c1-30(2,3)23-16-22(17-24(18-23)31(4,5)6)28-19-27(20-8-10-21(11-9-20)29(34)35)32-33(28)25-12-14-26(15-13-25)38(7,36)37/h8-19H,1-7H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 461 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay |
Bioorg Med Chem Lett 26: 3274-3277 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.056
BindingDB Entry DOI: 10.7270/Q2FF3V9N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290081
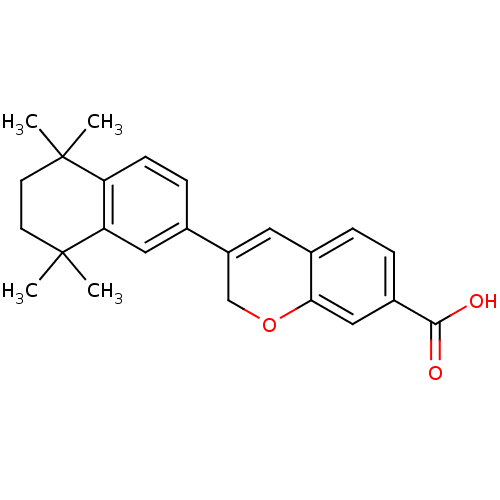
(3-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1=Cc2ccc(cc2OC1)C(O)=O |t:16| Show InChI InChI=1S/C24H26O3/c1-23(2)9-10-24(3,4)20-12-15(7-8-19(20)23)18-11-16-5-6-17(22(25)26)13-21(16)27-14-18/h5-8,11-13H,9-10,14H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 487 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2289-2294 (1997)
Article DOI: 10.1016/S0960-894X(97)00405-8
BindingDB Entry DOI: 10.7270/Q2HD7W4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048281
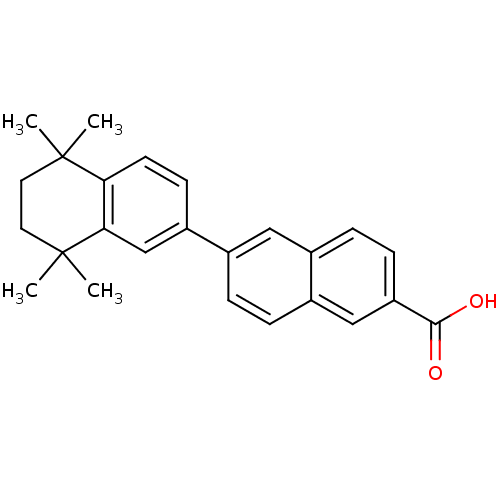
(5',5',8',8'-Tetramethyl-5',6',7',8'-tetrahydro-[2,...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc2cc(ccc2c1)C(O)=O Show InChI InChI=1S/C25H26O2/c1-24(2)11-12-25(3,4)22-15-19(9-10-21(22)24)17-5-6-18-14-20(23(26)27)8-7-16(18)13-17/h5-10,13-15H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048281

(5',5',8',8'-Tetramethyl-5',6',7',8'-tetrahydro-[2,...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc2cc(ccc2c1)C(O)=O Show InChI InChI=1S/C25H26O2/c1-24(2)11-12-25(3,4)22-15-19(9-10-21(22)24)17-5-6-18-14-20(23(26)27)8-7-16(18)13-17/h5-10,13-15H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2289-2294 (1997)
Article DOI: 10.1016/S0960-894X(97)00405-8
BindingDB Entry DOI: 10.7270/Q2HD7W4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048278
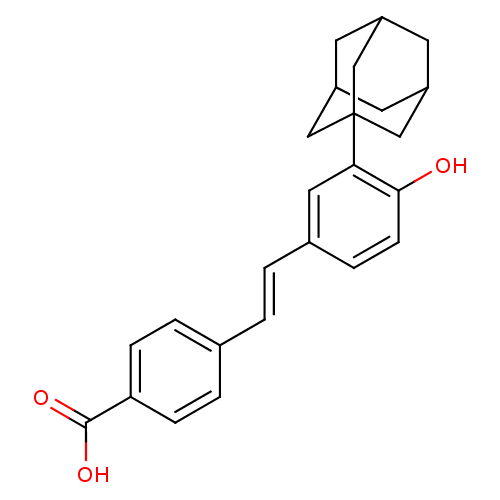
(4-[(E)-2-(3-Adamantan-1-yl-4-hydroxy-phenyl)-vinyl...)Show SMILES OC(=O)c1ccc(\C=C\c2ccc(O)c(c2)C23CC4CC(CC(C4)C2)C3)cc1 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:20:17:24.22.23,21:22:17:25.20.19| Show InChI InChI=1S/C25H26O3/c26-23-8-5-17(2-1-16-3-6-21(7-4-16)24(27)28)12-22(23)25-13-18-9-19(14-25)11-20(10-18)15-25/h1-8,12,18-20,26H,9-11,13-15H2,(H,27,28)/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048284
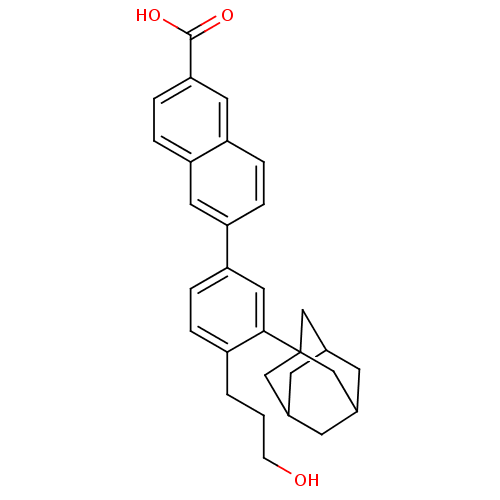
(6-[3-Adamantan-1-yl-4-(3-hydroxy-propyl)-phenyl]-n...)Show SMILES OCCCc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc2cc(ccc2c1)C(O)=O |TLB:13:14:18:11.12.17,17:16:19:11.12.13,17:12:19:18.16.15,THB:13:12:18:19.14.15| Show InChI InChI=1S/C30H32O3/c31-9-1-2-22-3-4-26(24-5-6-25-14-27(29(32)33)8-7-23(25)13-24)15-28(22)30-16-19-10-20(17-30)12-21(11-19)18-30/h3-8,13-15,19-21,31H,1-2,9-12,16-18H2,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 695 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290082
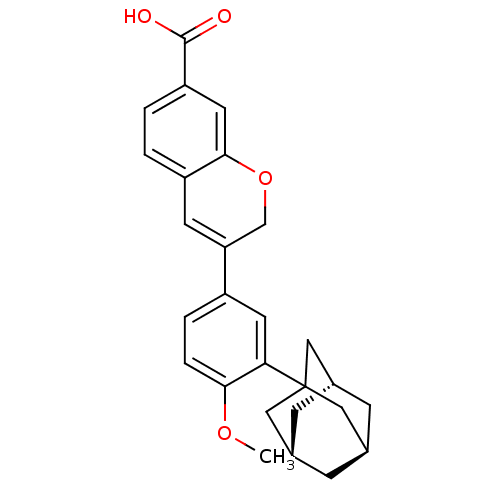
(3-(3-Adamantan-1-yl-4-methoxy-phenyl)-2H-chromene-...)Show SMILES COc1ccc(cc1C12C[C@H]3C[C@H](C[C@H](C3)C1)C2)C1=Cc2ccc(cc2OC1)C(O)=O |t:22,TLB:15:10:17:16.14.13,15:14:17:9.10.11| Show InChI InChI=1S/C27H28O4/c1-30-24-5-4-19(22-9-20-2-3-21(26(28)29)11-25(20)31-15-22)10-23(24)27-12-16-6-17(13-27)8-18(7-16)14-27/h2-5,9-11,16-18H,6-8,12-15H2,1H3,(H,28,29)/t16-,17+,18-,27? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 764 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2289-2294 (1997)
Article DOI: 10.1016/S0960-894X(97)00405-8
BindingDB Entry DOI: 10.7270/Q2HD7W4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290078
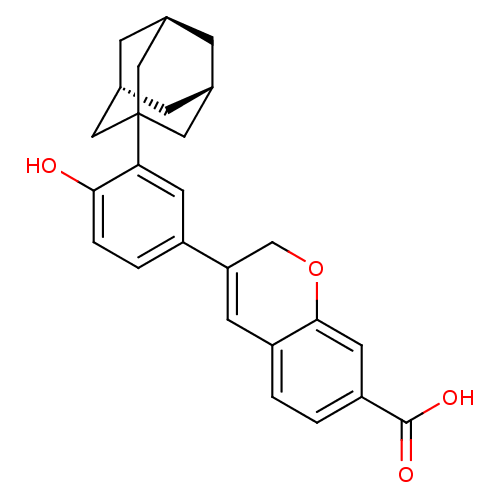
(3-(3-Adamantan-1-yl-4-hydroxy-phenyl)-2H-chromene-...)Show SMILES OC(=O)c1ccc2C=C(COc2c1)c1ccc(O)c(c1)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |c:7,TLB:27:22:29:28.26.25,27:26:29:21.22.23| Show InChI InChI=1S/C26H26O4/c27-23-4-3-18(21-8-19-1-2-20(25(28)29)10-24(19)30-14-21)9-22(23)26-11-15-5-16(12-26)7-17(6-15)13-26/h1-4,8-10,15-17,27H,5-7,11-14H2,(H,28,29)/t15-,16+,17-,26? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 821 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2289-2294 (1997)
Article DOI: 10.1016/S0960-894X(97)00405-8
BindingDB Entry DOI: 10.7270/Q2HD7W4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50048290
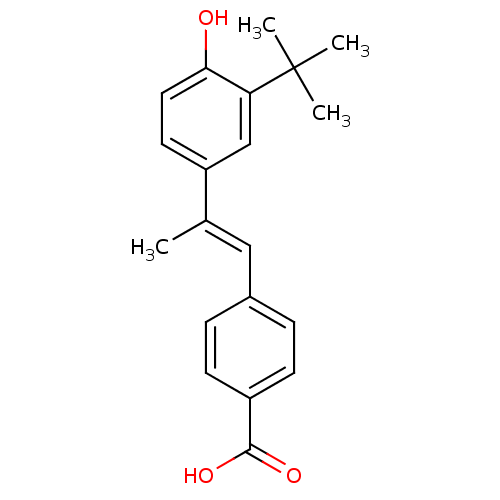
(4-[(E)-2-(3-tert-Butyl-4-hydroxy-phenyl)-propenyl]...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc(O)c(c1)C(C)(C)C Show InChI InChI=1S/C20H22O3/c1-13(11-14-5-7-15(8-6-14)19(22)23)16-9-10-18(21)17(12-16)20(2,3)4/h5-12,21H,1-4H3,(H,22,23)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 834 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIRD GALDERMA
Curated by ChEMBL
| Assay Description
Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligand |
J Med Chem 38: 4993-5006 (1996)
BindingDB Entry DOI: 10.7270/Q20G3J8S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061618
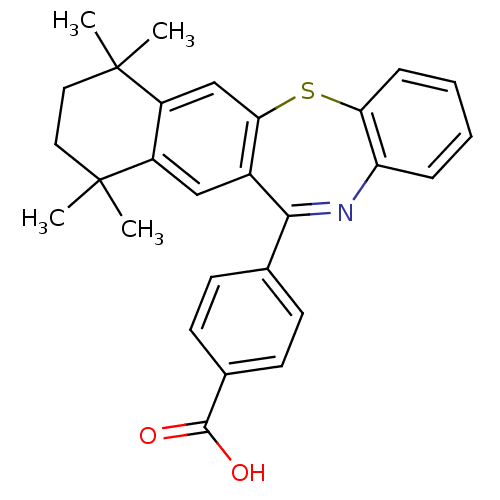
(4-(7,7,10,10-Tetramethyl-7,8,9,10-tetrahydro-5-thi...)Show SMILES CC1(C)CCC(C)(C)c2cc3c(Sc4ccccc4N=C3c3ccc(cc3)C(O)=O)cc12 |c:20| Show InChI InChI=1S/C28H27NO2S/c1-27(2)13-14-28(3,4)21-16-24-19(15-20(21)27)25(17-9-11-18(12-10-17)26(30)31)29-22-7-5-6-8-23(22)32-24/h5-12,15-16H,13-14H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Synergistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50033079
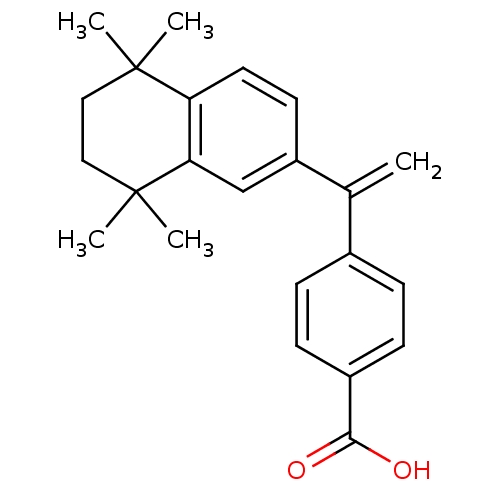
(4-[1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=C)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H26O2/c1-15(16-6-8-17(9-7-16)21(24)25)18-10-11-19-20(14-18)23(4,5)13-12-22(19,2)3/h6-11,14H,1,12-13H2,2-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 944 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to RAR alpha receptor |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061617
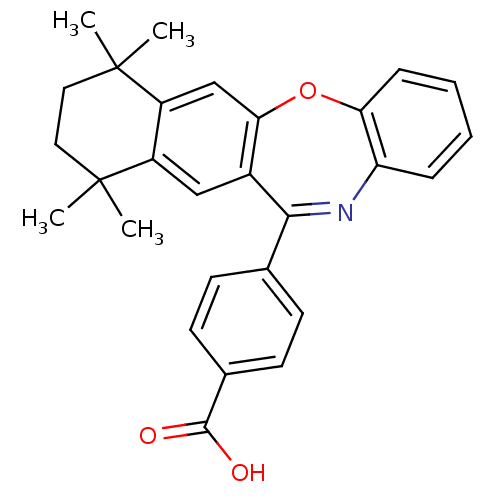
(4-(7,7,10,10-Tetramethyl-7,8,9,10-tetrahydro-5-oxa...)Show SMILES CC1(C)CCC(C)(C)c2cc3c(Oc4ccccc4N=C3c3ccc(cc3)C(O)=O)cc12 |c:20| Show InChI InChI=1S/C28H27NO3/c1-27(2)13-14-28(3,4)21-16-24-19(15-20(21)27)25(17-9-11-18(12-10-17)26(30)31)29-22-7-5-6-8-23(22)32-24/h5-12,15-16H,13-14H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Synergistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143821
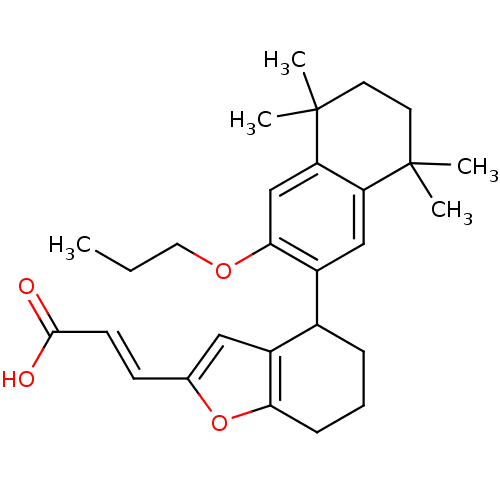
((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...)Show SMILES CCCOc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C28H36O4/c1-6-14-31-25-17-23-22(27(2,3)12-13-28(23,4)5)16-21(25)19-8-7-9-24-20(19)15-18(32-24)10-11-26(29)30/h10-11,15-17,19H,6-9,12-14H2,1-5H3,(H,29,30)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143826
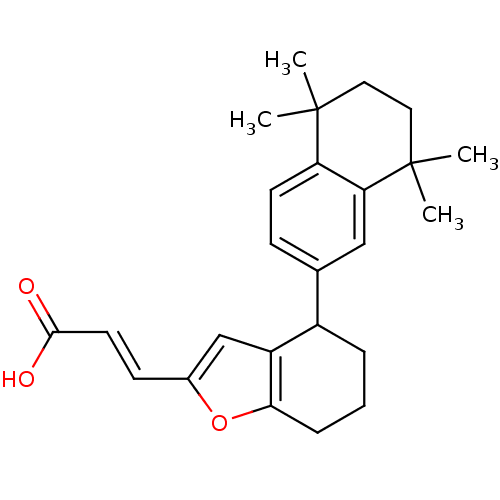
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
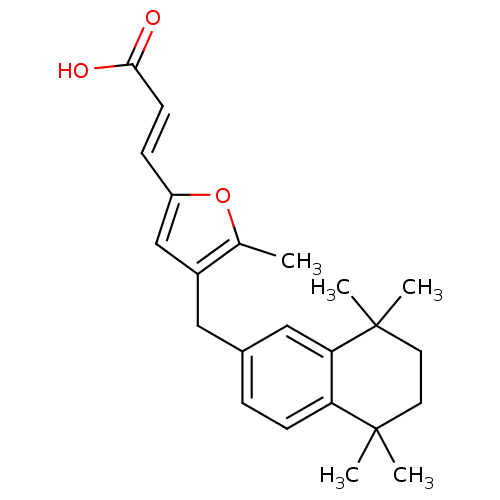
(Homo sapiens (Human)) | BDBM50143823
((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...)Show SMILES Cc1oc(\C=C\C(O)=O)cc1Cc1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O3/c1-15-17(14-18(26-15)7-9-21(24)25)12-16-6-8-19-20(13-16)23(4,5)11-10-22(19,2)3/h6-9,13-14H,10-12H2,1-5H3,(H,24,25)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143827

((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...)Show SMILES COc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O4/c1-25(2)11-12-26(3,4)21-15-23(29-5)19(14-20(21)25)17-7-6-8-22-18(17)13-16(30-22)9-10-24(27)28/h9-10,13-15,17H,6-8,11-12H2,1-5H3,(H,27,28)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
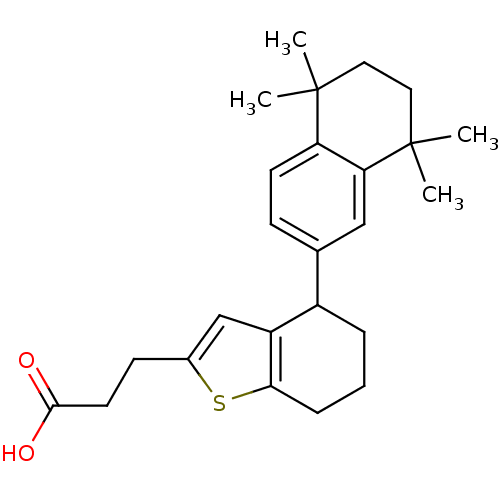
(Homo sapiens (Human)) | BDBM50143828
(3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2sc(CCC(O)=O)cc12 Show InChI InChI=1S/C25H32O2S/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8,10,14-15,18H,5-7,9,11-13H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
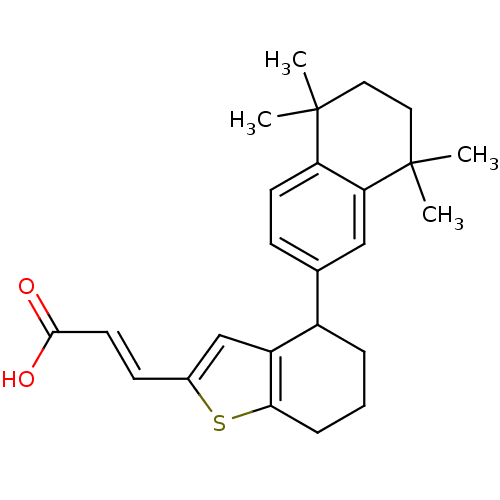
(Homo sapiens (Human)) | BDBM50143833
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2sc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O2S/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143825
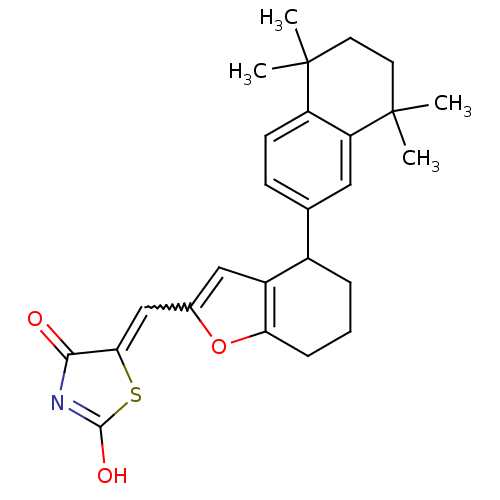
(5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(C=C3SC(O)=NC3=O)cc12 |w:21.22,c:27| Show InChI InChI=1S/C26H29NO3S/c1-25(2)10-11-26(3,4)20-12-15(8-9-19(20)25)17-6-5-7-21-18(17)13-16(30-21)14-22-23(28)27-24(29)31-22/h8-9,12-14,17H,5-7,10-11H2,1-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143830

(4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(cc12)C(O)=O Show InChI InChI=1S/C23H28O3/c1-22(2)10-11-23(3,4)18-12-14(8-9-17(18)22)15-6-5-7-19-16(15)13-20(26-19)21(24)25/h8-9,12-13,15H,5-7,10-11H2,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143831
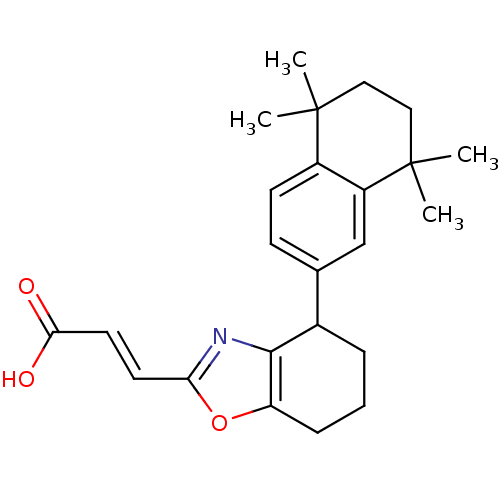
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)nc12 Show InChI InChI=1S/C24H29NO3/c1-23(2)12-13-24(3,4)18-14-15(8-9-17(18)23)16-6-5-7-19-22(16)25-20(28-19)10-11-21(26)27/h8-11,14,16H,5-7,12-13H2,1-4H3,(H,26,27)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143835
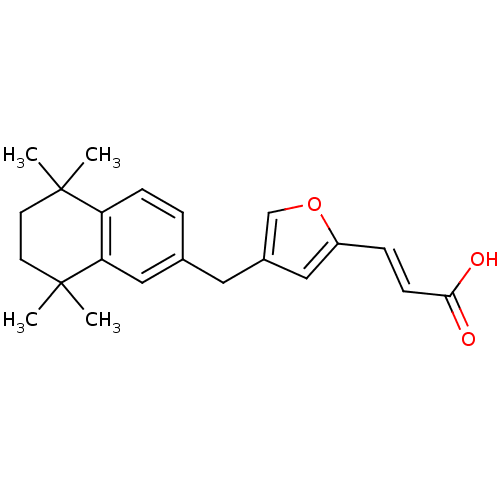
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(Cc3coc(\C=C\C(O)=O)c3)ccc12 Show InChI InChI=1S/C22H26O3/c1-21(2)9-10-22(3,4)19-13-15(5-7-18(19)21)11-16-12-17(25-14-16)6-8-20(23)24/h5-8,12-14H,9-11H2,1-4H3,(H,23,24)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143832

((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES Cc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O3/c1-16-13-21-22(26(4,5)12-11-25(21,2)3)15-19(16)18-7-6-8-23-20(18)14-17(29-23)9-10-24(27)28/h9-10,13-15,18H,6-8,11-12H2,1-5H3,(H,27,28)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50129720

((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34F2O3/c1-16(12-22(28)29)10-9-11-17(2)19-13-18(24(3,4)5)14-20(25(6,7)8)23(19)30-15-21(26)27/h9-14,21H,15H2,1-8H3,(H,28,29)/b10-9+,16-12+,17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic acid receptor alpha by [3H]-ATRA displacement. |
Bioorg Med Chem Lett 14: 1593-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.089
BindingDB Entry DOI: 10.7270/Q2B857JF |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290186
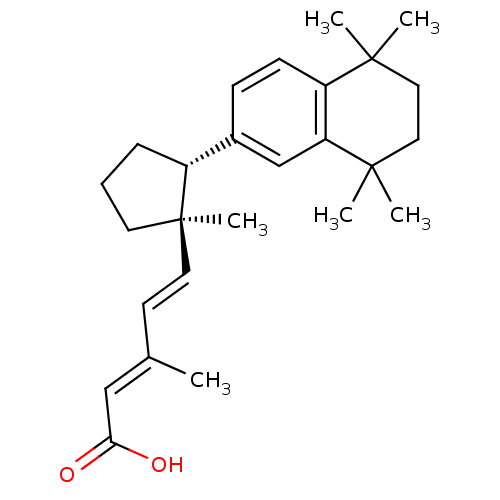
((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@]1(C)CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H36O2/c1-18(16-23(27)28)11-13-26(6)12-7-8-20(26)19-9-10-21-22(17-19)25(4,5)15-14-24(21,2)3/h9-11,13,16-17,20H,7-8,12,14-15H2,1-6H3,(H,27,28)/b13-11+,18-16+/t20-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290187
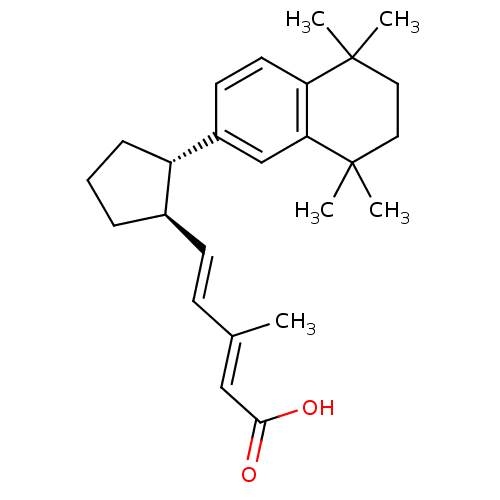
((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...)Show SMILES C\C(\C=C\[C@H]1CCC[C@@H]1c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34O2/c1-17(15-23(26)27)9-10-18-7-6-8-20(18)19-11-12-21-22(16-19)25(4,5)14-13-24(21,2)3/h9-12,15-16,18,20H,6-8,13-14H2,1-5H3,(H,26,27)/b10-9+,17-15+/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to retinoic acid receptor RAR alpha |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data