

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409275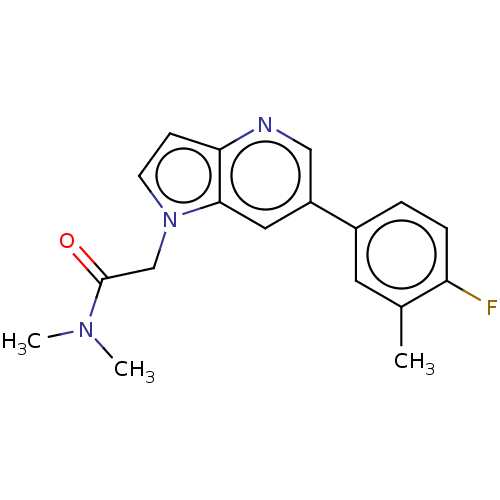 (2-[6-(4-Fluoro-3-methyl-phenyl)pyrrolo[3,2-b]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436977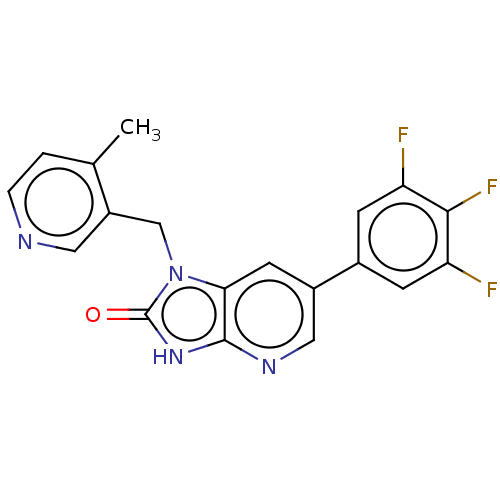 (1-[(4-Methyl-3-pyridyl)methyl]-6-(3,4,5-trifluorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436994 (6-(3,4-Difluorophenyl)-1-(2-oxobutyl)-3H-imidazo[4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124908 (CHEMBL159560 | N-(2-Methoxy-benzyl)-4-trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the response to NMDA glutamate/glycine receptor NR2B subtype was determined using FLIPR assay | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124909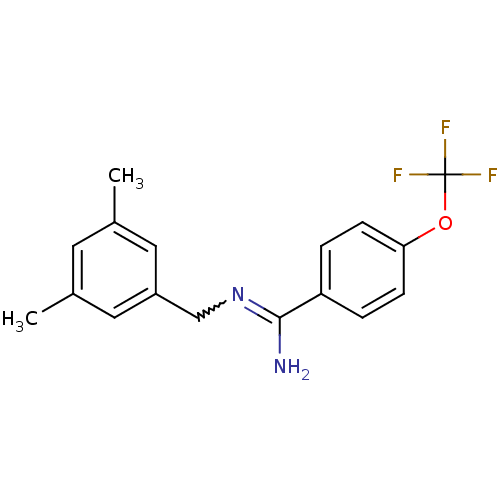 (CHEMBL159744 | N-(3,5-Dimethyl-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the response to NMDA glutamate/glycine receptor NR2B subtype was determined using FLIPR assay | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198665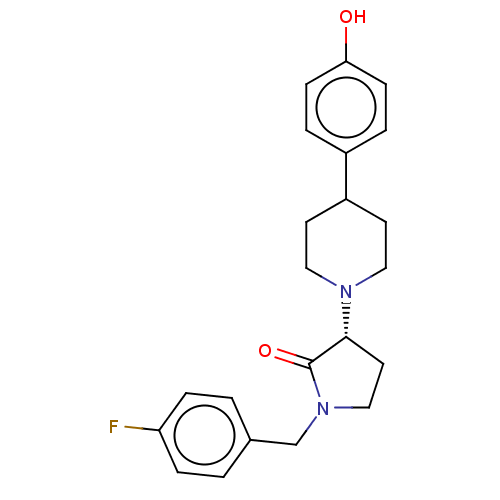 (US9221796, 2b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to GluN2B receptor in human cortex | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436678 (2-[6-(5-Chloro-2-thienyl)-3-methyl-2-oxo-imidazo[4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50212684 (2-(2-methoxy-benzyl)-5-trifluoromethoxy-2,3-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at NMDA NR2B receptor assessed as calcium flux | Bioorg Med Chem Lett 17: 3997-4000 (2007) Article DOI: 10.1016/j.bmcl.2007.04.084 BindingDB Entry DOI: 10.7270/Q21J99GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436762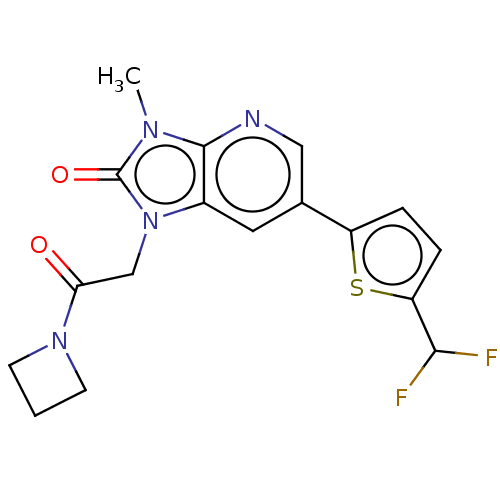 (1-[2-(Azetidin-1-yl)-2-oxo-ethyl]-6-[5-(difluorome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM437086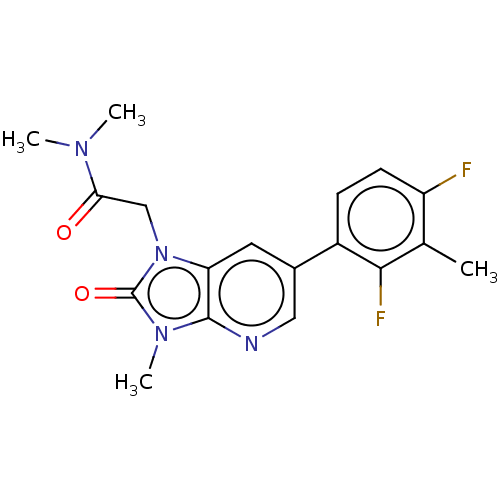 (2-[6-(2,4-Difluoro-3-methyl-phenyl)-3-methyl-2-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409513 (2-[6-(5-Chloro-2-thienyl)pyrrolo[3,2-b]pyridin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436860 (N-(3,3-Difluorocyclobutyl)-2-[2-oxo-6-[3-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436768 (6-[5-(Difluoromethyl)-3-thienyl]-1-[2-(3-fluoroaze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50379671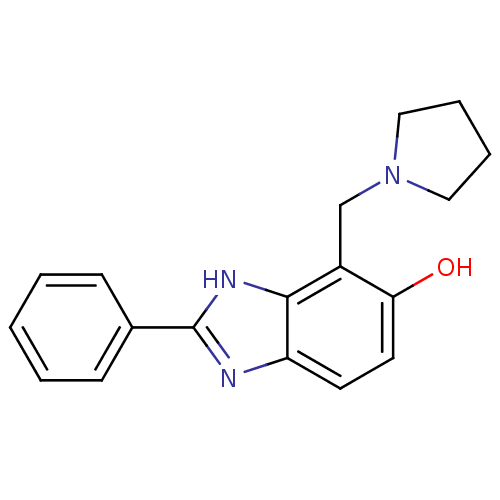 (CHEMBL2013197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-Ro256981 from human NR2B receptor | Bioorg Med Chem Lett 22: 2620-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.108 BindingDB Entry DOI: 10.7270/Q2KD1ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50379688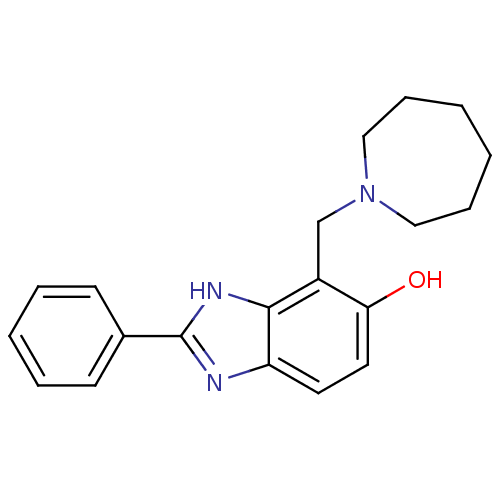 (CHEMBL2013196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-Ro256981 from human NR2B receptor | Bioorg Med Chem Lett 22: 2620-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.108 BindingDB Entry DOI: 10.7270/Q2KD1ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50379670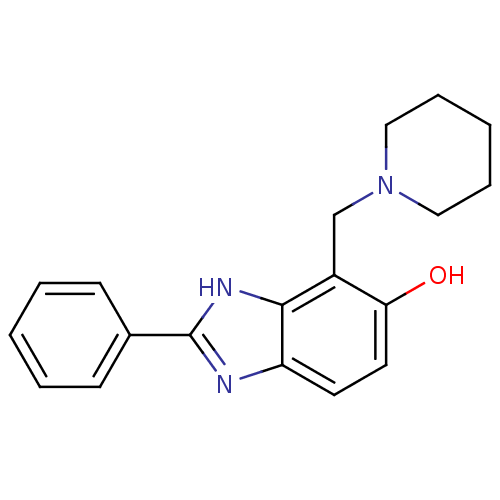 (CHEMBL2013195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-Ro256981 from human NR2B receptor | Bioorg Med Chem Lett 22: 2620-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.108 BindingDB Entry DOI: 10.7270/Q2KD1ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM437073 (1-[2-(Azetidin-1-yl)-2-oxo-ethyl]-6-(2,4-difluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM437111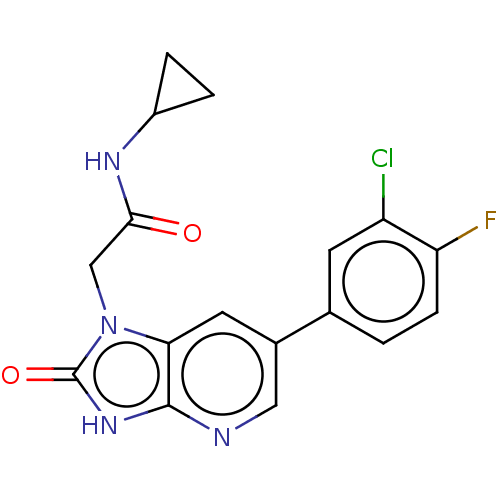 (2-[6-(3-Chloro-4-fluoro-phenyl)-2-oxo-3H-imidazo[4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124923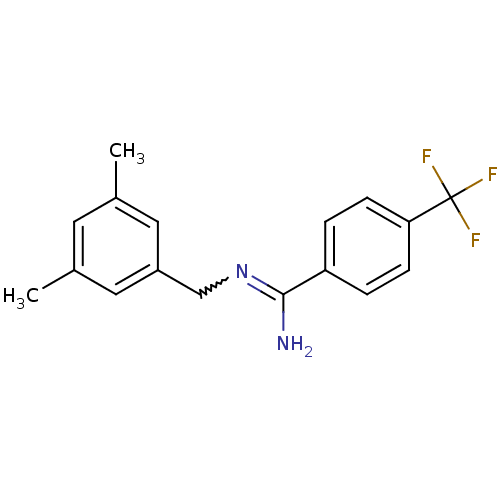 (CHEMBL162080 | N-(3,5-Dimethyl-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the response to NMDA glutamate/glycine receptor NR2B subtype was determined using FLIPR assay | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436719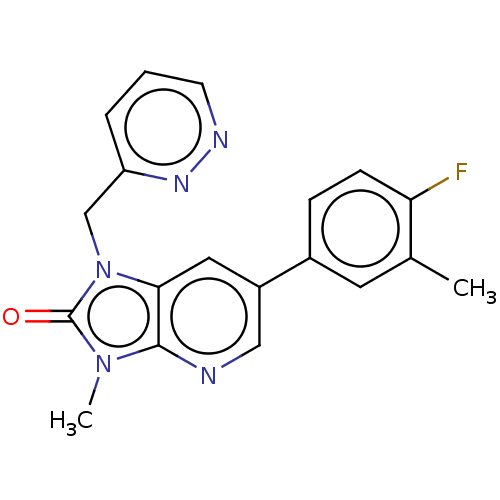 (6-(4-Fluoro-3-methyl-phenyl)-3-methyl-1-(pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50301369 ((+)--5-Hydroxy-3-phenyl-5H-benzo[4,5]cyclohepta[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity against NR2B receptor expressed in mouse Ltk cells assessed as inhibition of calcium influx by FLIPR assay | Bioorg Med Chem Lett 19: 5132-5 (2009) Article DOI: 10.1016/j.bmcl.2009.07.028 BindingDB Entry DOI: 10.7270/Q2X34XJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436940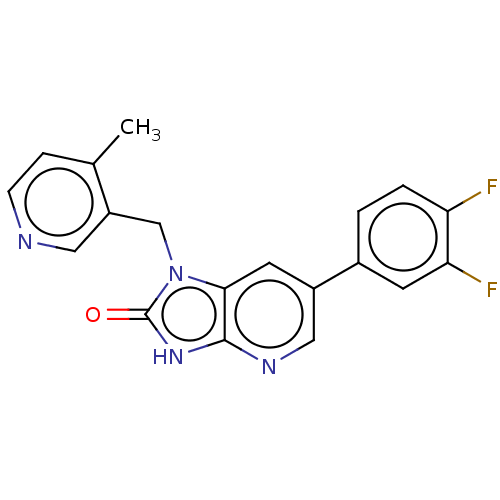 (6-(3,4-Difluorophenyl)-1-[(4-methyl-3-pyridyl)meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM437076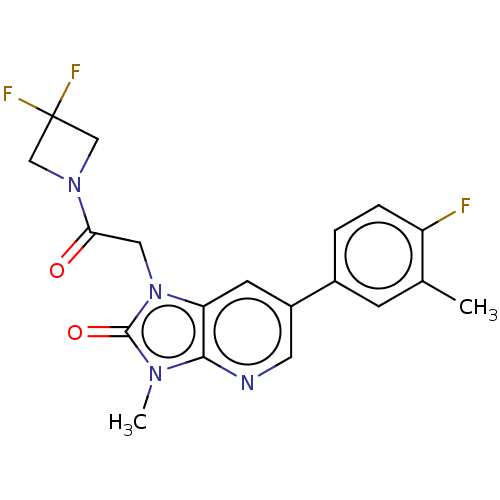 (1-(2-(3,3-Difluoroazetidin-1-yl)-2-oxoethyl)-6-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50080029 (4-((1R,2S)-3-(4-benzylpiperidin-1-yl)-1-hydroxy-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Negative allosteric modulation of GluN2B receptor (unknown origin) expressed in xenopus laevis oocytes assessed as reduction in glycine-induced chann... | J Med Chem 62: 3-23 (2019) Article DOI: 10.1021/acs.jmedchem.7b01640 BindingDB Entry DOI: 10.7270/Q2N019RG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436736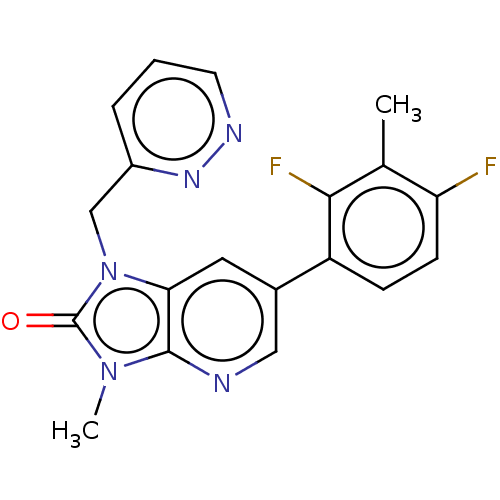 (6-(2,4-Difluoro-3-methyl-phenyl)-3-methyl-1-(pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM617021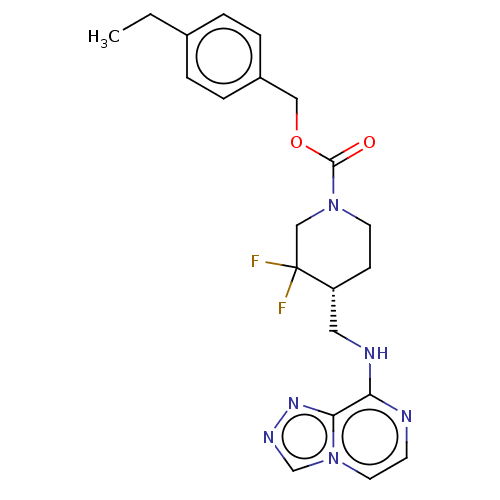 (US11752155, Compound E1-1.5B') | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM617021 (US11752155, Compound E1-1.5B') | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM617021 (US11752155, Compound E1-1.5B') | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124917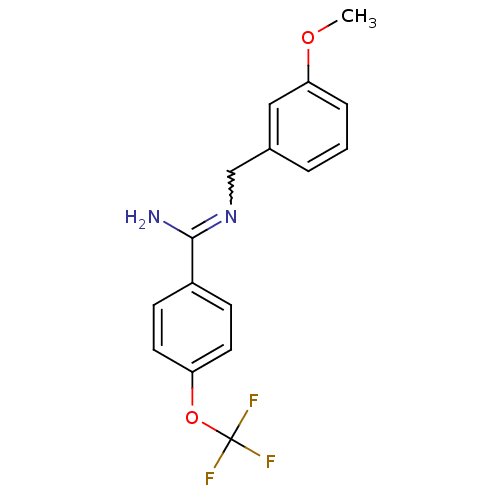 (CHEMBL349727 | N-(3-Methoxy-benzyl)-4-trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM437085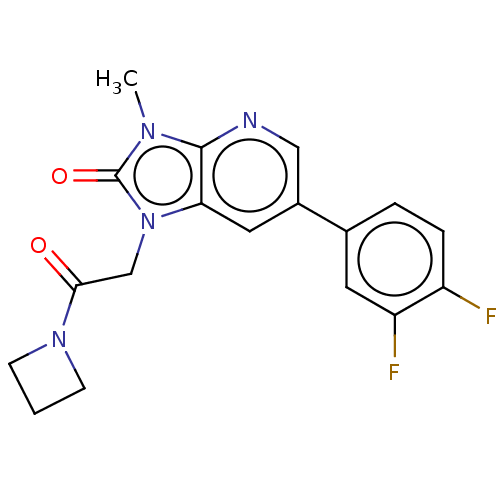 (1-[2-(Azetidin-1-yl)-2-oxo-ethyl]-6-(3,4-difluorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM437079 (1-[2-(3,3-Difluoroazetidin-1-yl)-2-oxo-ethyl]-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409515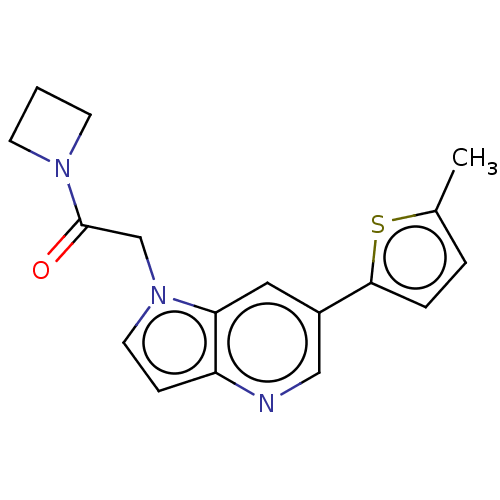 (1-(Azetidin-1-yl)-2-[6-(5-methyl-2-thienyl)pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409512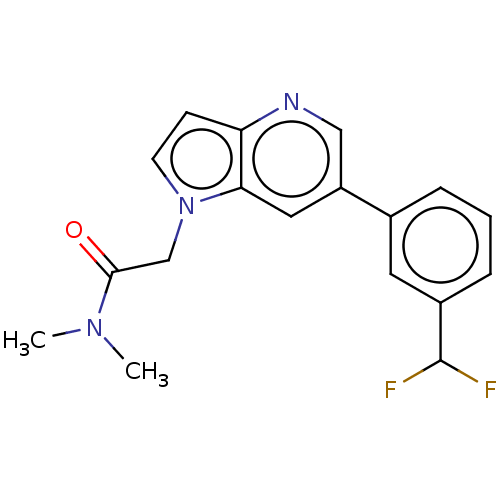 (2-[6-[3-(Difluoromethyl)phenyl]pyrrolo[3,2-b]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409735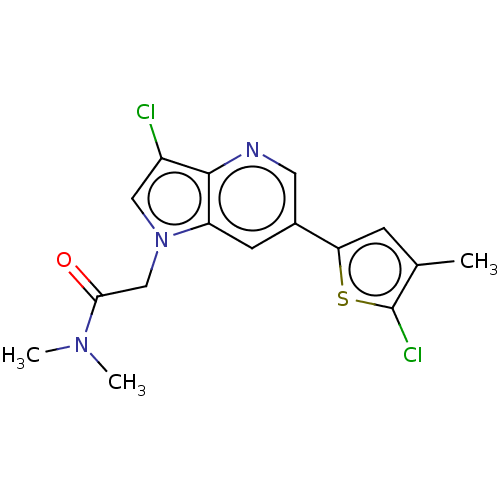 (2-[3-Chloro-6-(5-chloro-4-methyl-2-thienyl)pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436679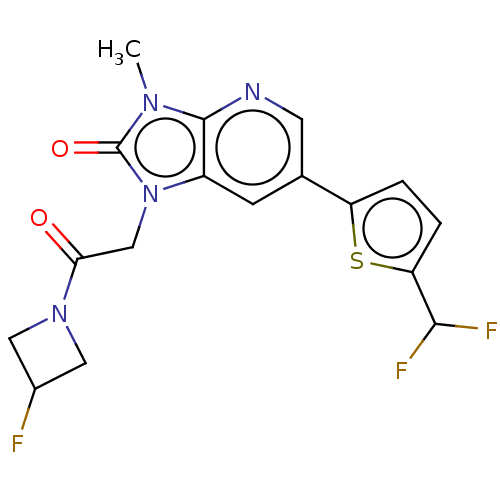 (6-[5-(Difluoromethyl)-2-thienyl]-1-[2-(3-fluoroaze...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM436696 (1-[2-(Azetidin-1-yl)-2-oxo-ethyl]-3-methyl-6-[5-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description NMDA receptors are ion channels that are highly permeable to Ca2+ ions, rendering it possible to monitor NMDA receptor function using cell-based calc... | US Patent US10617676 (2020) BindingDB Entry DOI: 10.7270/Q2CJ8HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409743 (2-[6-(3,5-Difluorophenyl)-3-(trifluoromethyl)pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM409623 (2-[6-(3-Chlorophenyl)-3-fluoro-pyrrolo[3,2-b]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay depends on the binding of a tracer to the GluN2B subunit-containing NMDA receptors and the ability of the test compounds to displace such b... | US Patent US10377753 (2019) BindingDB Entry DOI: 10.7270/Q2CJ8GVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM388296 (US10294230, Compound E1-2.2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM388296 (US10294230, Compound E1-2.2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM435144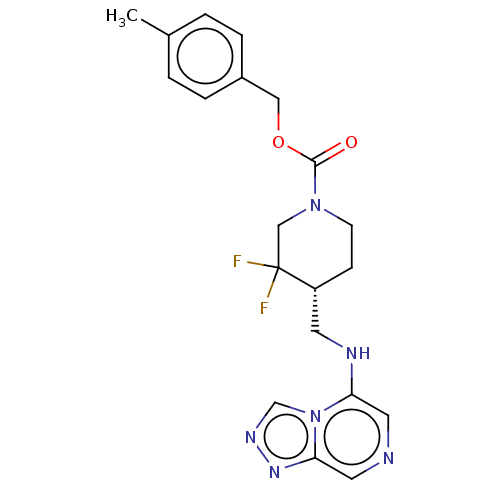 (US10584127, Compound E1-2.2 | US11136328, Compound...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rugen Holdings (Cayman) Limited US Patent | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | US Patent US10584127 (2020) BindingDB Entry DOI: 10.7270/Q2Q242NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM435144 (US10584127, Compound E1-2.2 | US11136328, Compound...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H41VMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM435144 (US10584127, Compound E1-2.2 | US11136328, Compound...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H41VMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM435144 (US10584127, Compound E1-2.2 | US11136328, Compound...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H41VMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM617013 (US11752155, Compound E1-2.2B') | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM388296 (US10294230, Compound E1-2.2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2ZW1P6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM617013 (US11752155, Compound E1-2.2B') | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM617013 (US11752155, Compound E1-2.2B') | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM388301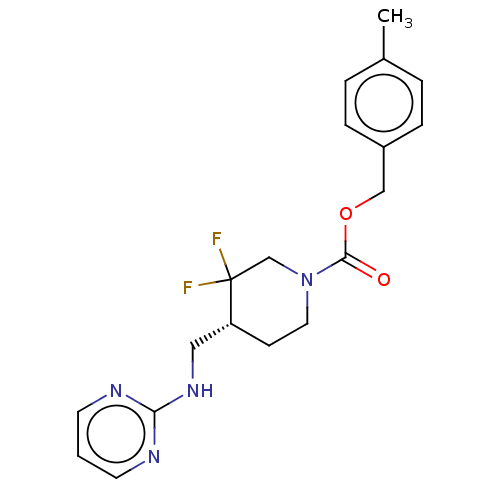 (US10294230, Compound E1-22.2 | US10584127, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM388301 (US10294230, Compound E1-22.2 | US10584127, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26T0RS5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2104 total ) | Next | Last >> |