

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human LPL expressed in COS7 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by sub... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 BindingDB Entry DOI: 10.7270/Q2TF01QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50138739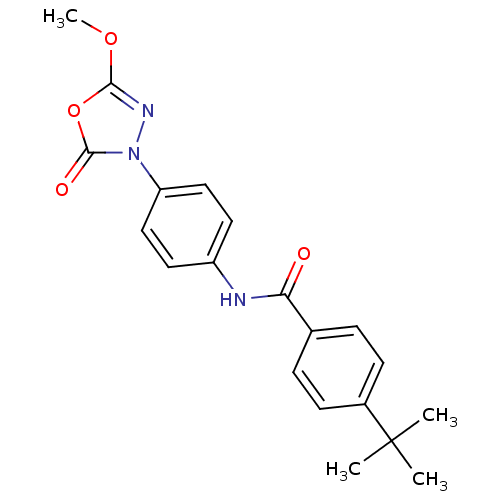 (4-tert-Butyl-N-[4-(5-methoxy-2-oxo-[1,3,4]oxadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was tested to inhibit lipoprotein lipase (LPL) | J Med Chem 47: 400-10 (2004) Article DOI: 10.1021/jm031004s BindingDB Entry DOI: 10.7270/Q2ZG6RN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM26134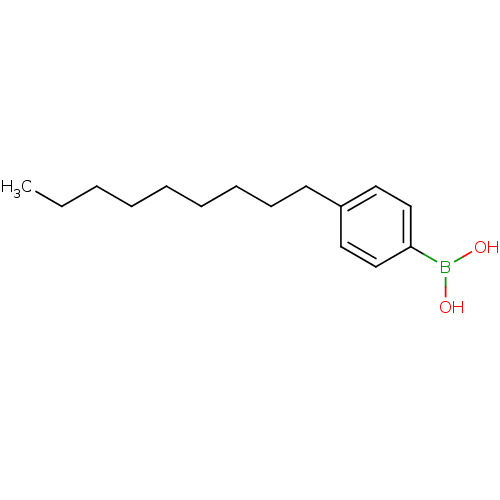 ((4-nonylphenyl)boranediol | Phenylboronic Acid, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364285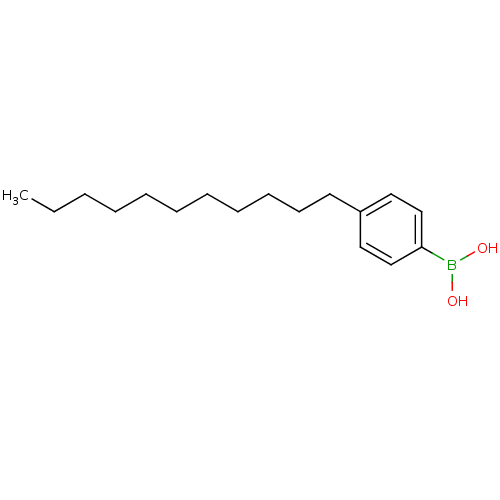 (CHEMBL1952298) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50433472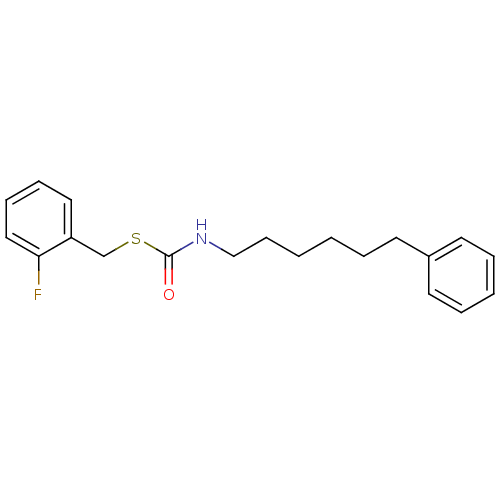 (CHEMBL2381069) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inhibition of human LPL expressed in HEK293 cells using PED-A1 as substrate by spectrophotometry | Bioorg Med Chem Lett 23: 2595-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.113 BindingDB Entry DOI: 10.7270/Q24T6KQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444153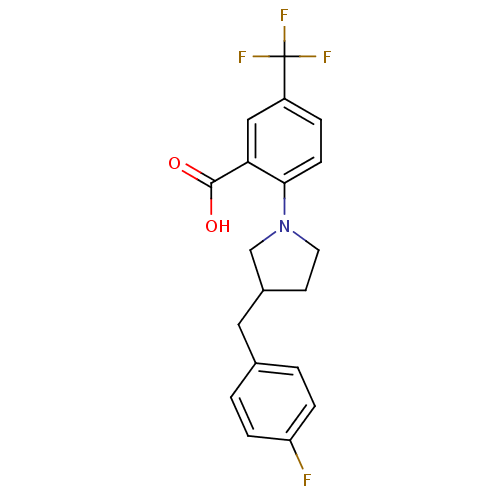 (CHEMBL3093304) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364283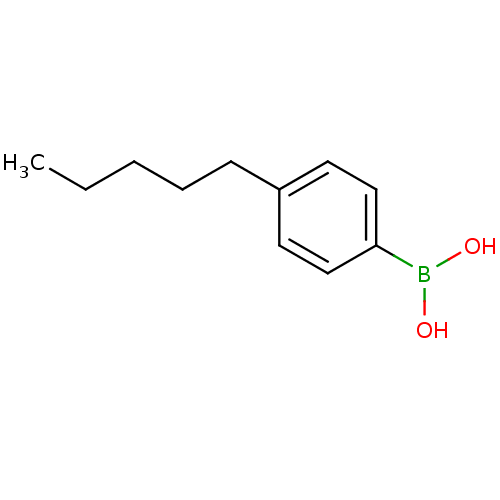 (CHEMBL1952296) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364295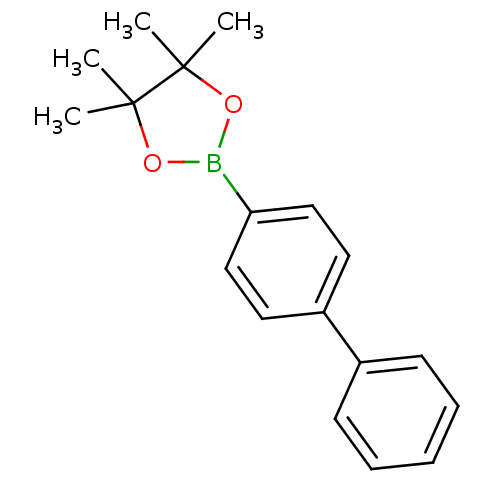 (CHEMBL1952307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364298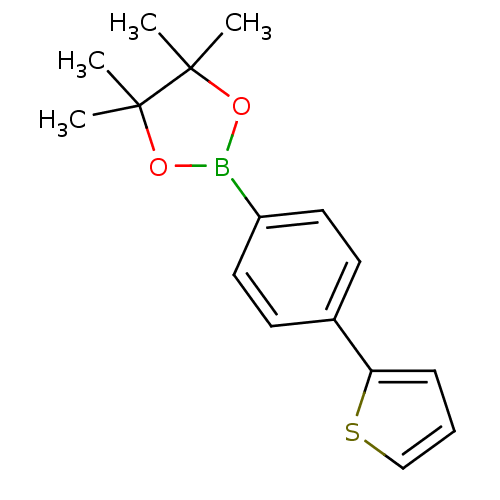 (CHEMBL1952310) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364282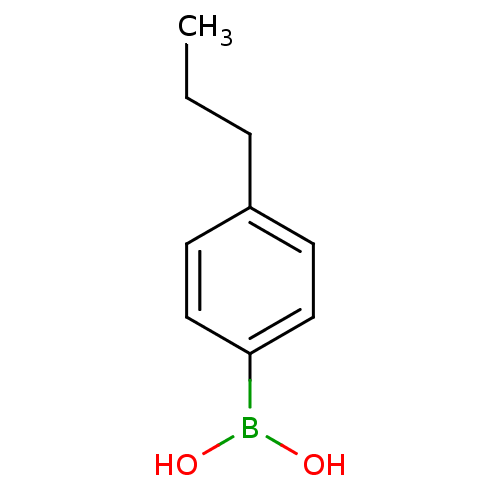 (CHEMBL1952295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50067895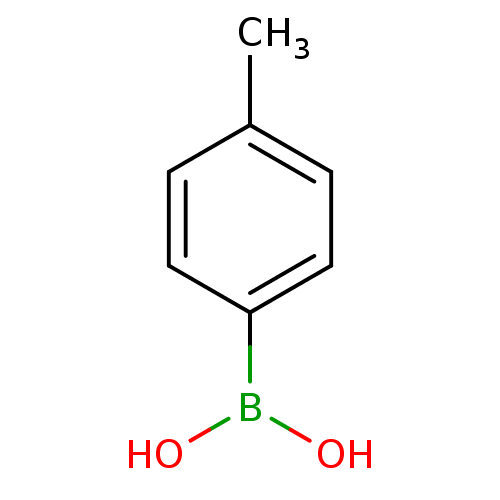 (CHEMBL140780 | Tolyl boronic acid | p-tolylboronic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364281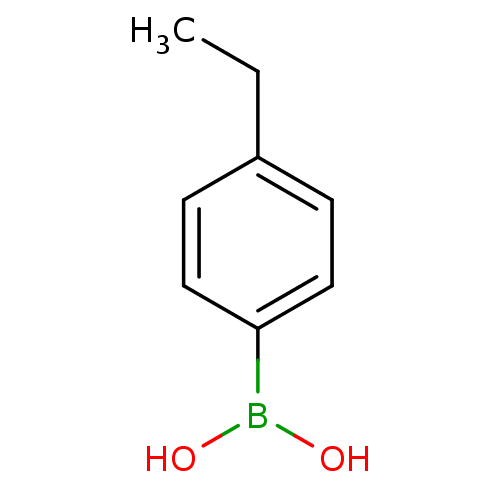 (CHEMBL1952294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364284 (CHEMBL1952297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364297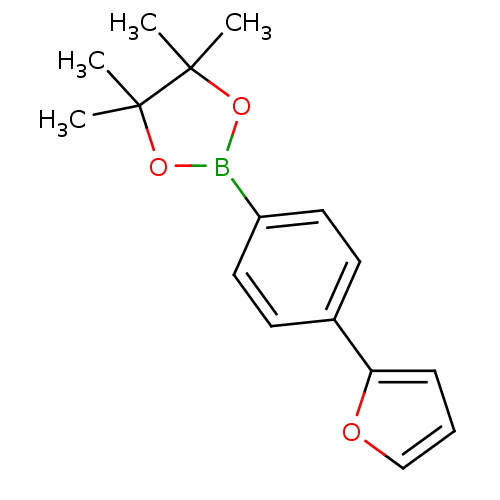 (CHEMBL1952309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444145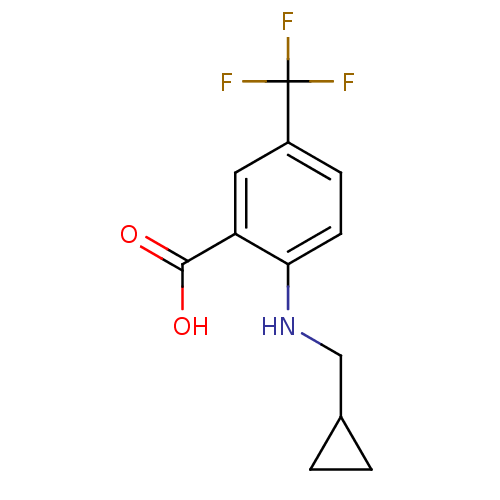 (CHEMBL3093405) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50138734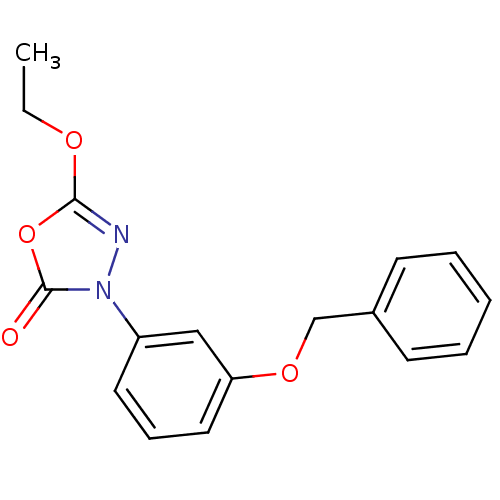 (3-(3-(benzyloxy)phenyl)-5-ethoxy-1,3,4-oxadiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was tested to inhibit lipoprotein lipase (LPL) | J Med Chem 47: 400-10 (2004) Article DOI: 10.1021/jm031004s BindingDB Entry DOI: 10.7270/Q2ZG6RN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364295 (CHEMBL1952307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50521226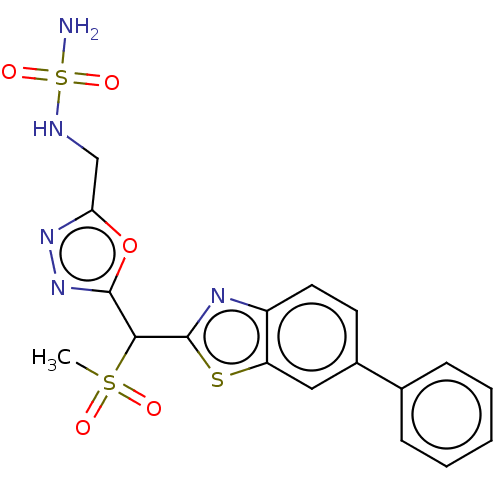 (CHEMBL4471545) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human LPL expressed in COS7 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by sub... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 BindingDB Entry DOI: 10.7270/Q2TF01QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364282 (CHEMBL1952295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364300 (CHEMBL1952312) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364283 (CHEMBL1952296) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364293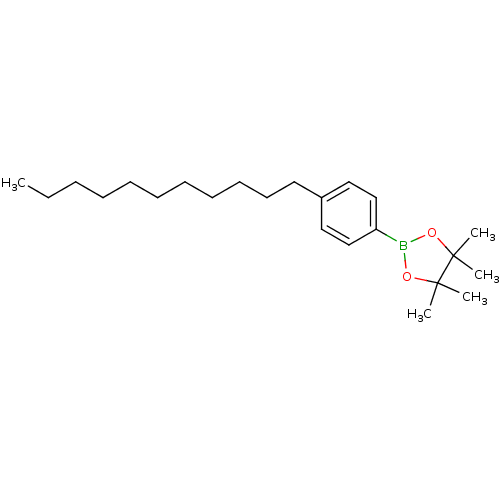 (CHEMBL1952305) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364301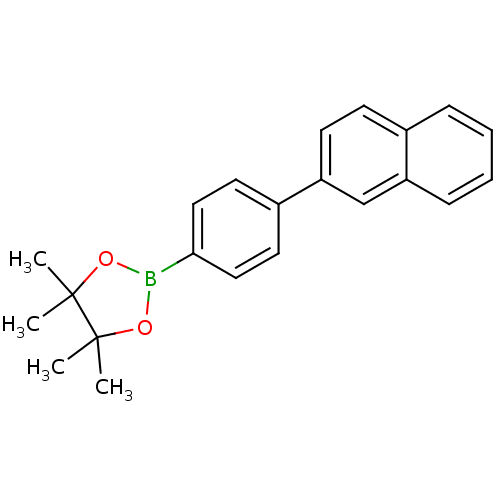 (CHEMBL1952313) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364299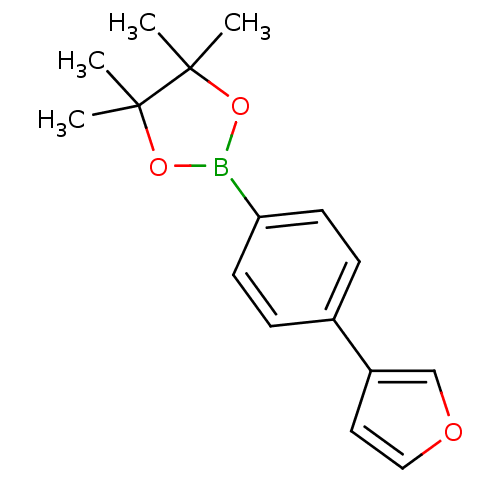 (CHEMBL1952311) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364292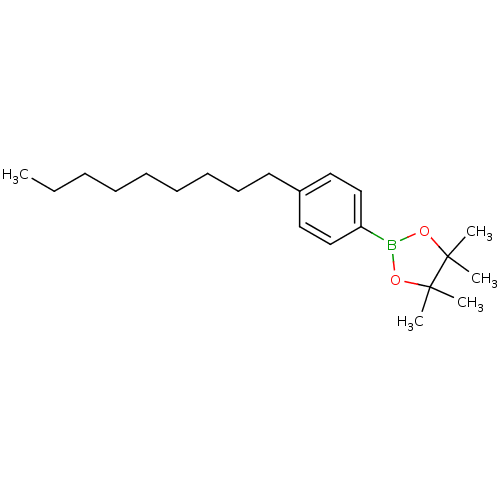 (CHEMBL1949700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364291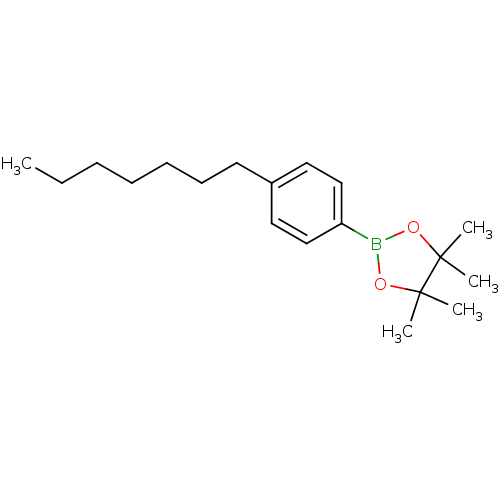 (CHEMBL1952304) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444152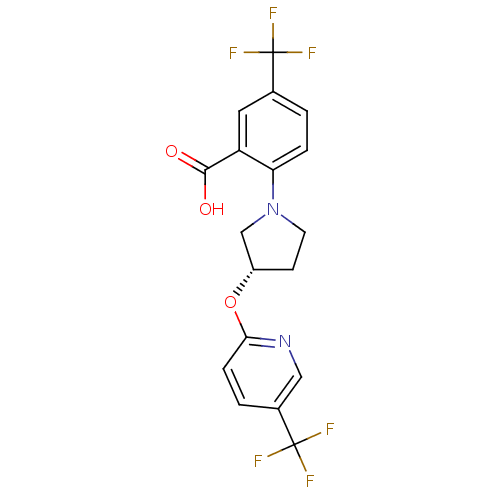 (CHEMBL3093305) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364281 (CHEMBL1952294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444138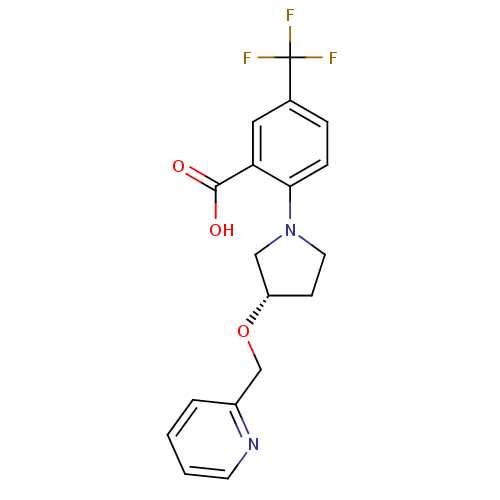 (CHEMBL3093392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364290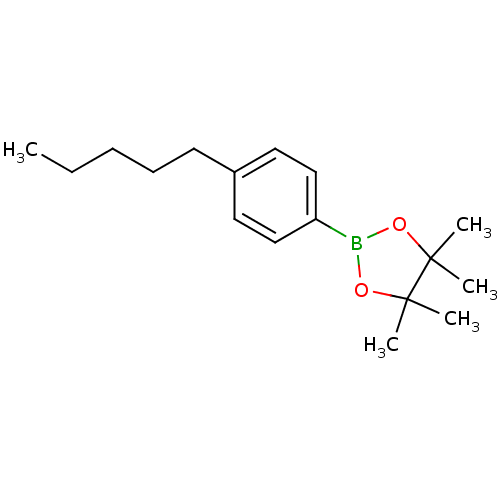 (CHEMBL1952303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364301 (CHEMBL1952313) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364289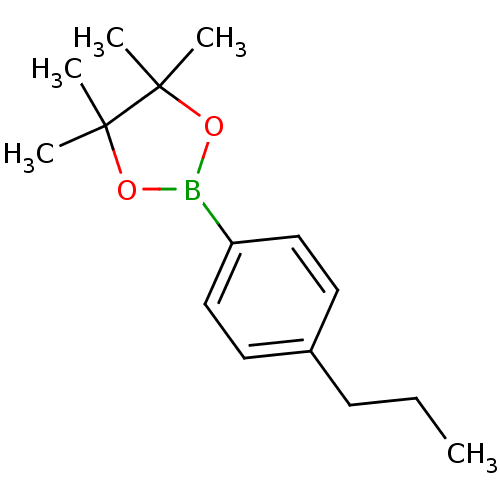 (CHEMBL1952302) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50067895 (CHEMBL140780 | Tolyl boronic acid | p-tolylboronic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364289 (CHEMBL1952302) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364284 (CHEMBL1952297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444151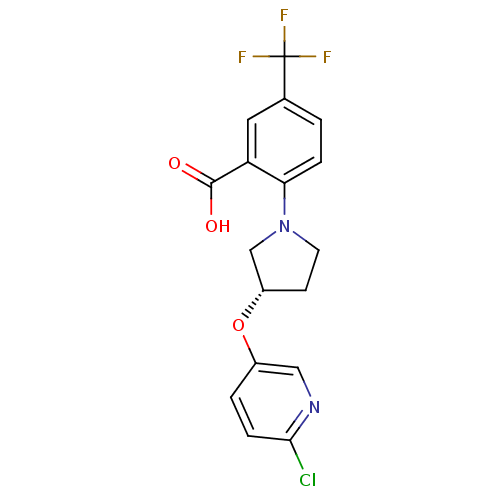 (CHEMBL3093306) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364296 (CHEMBL1952308) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444147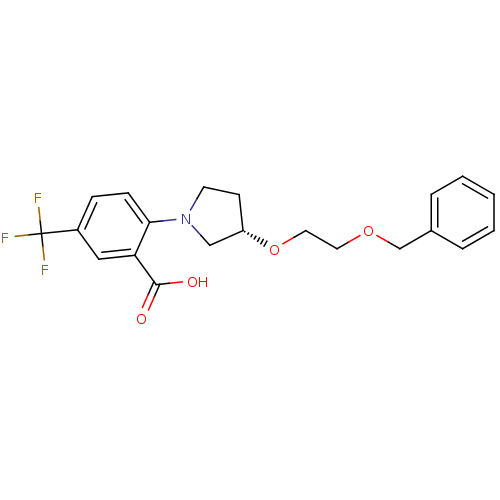 (CHEMBL3093396) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444150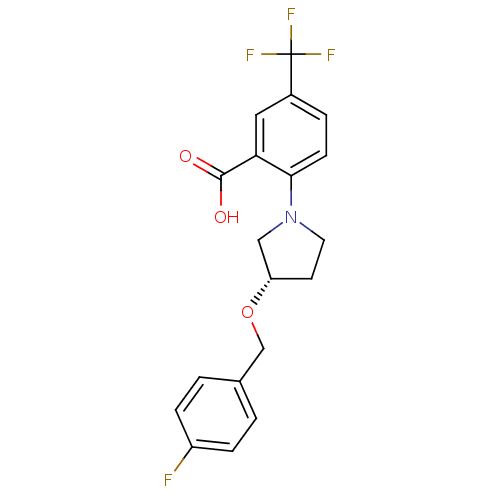 (CHEMBL3093307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444154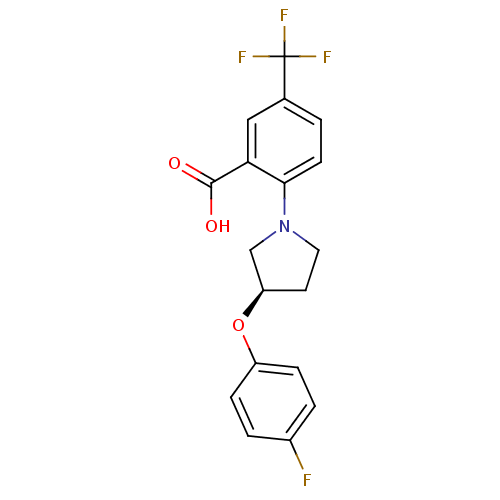 (CHEMBL3093303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444148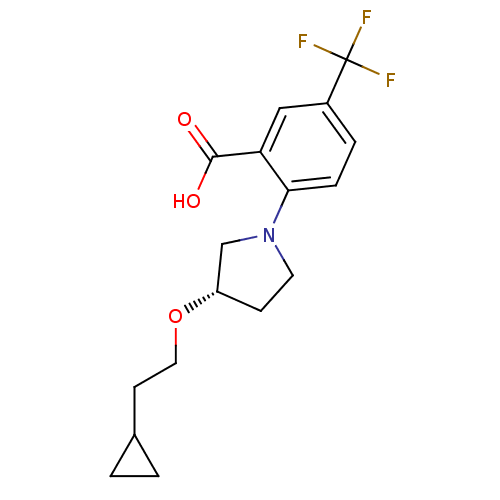 (CHEMBL3093309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364299 (CHEMBL1952311) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364297 (CHEMBL1952309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364300 (CHEMBL1952312) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50458639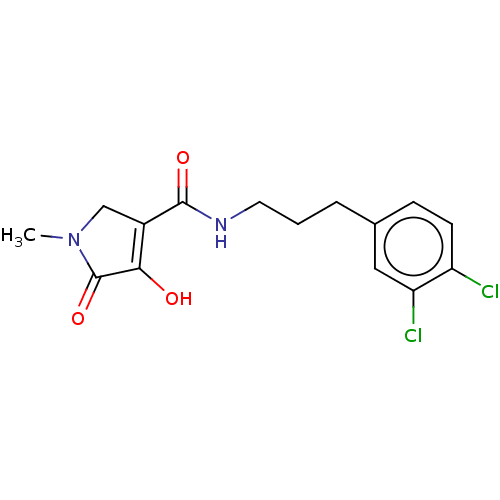 (CHEMBL4207216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human LPL expressed in African green monkey COS7 cells using DGGR/DMPG vesicles as substrate pretreated for 10 mins followe... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364298 (CHEMBL1952310) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed in HEK293 cells using bis-BD-PC and mono-BD-TG as substrate incubated for 10 mins prior to substrate... | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM26134 ((4-nonylphenyl)boranediol | Phenylboronic Acid, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50364294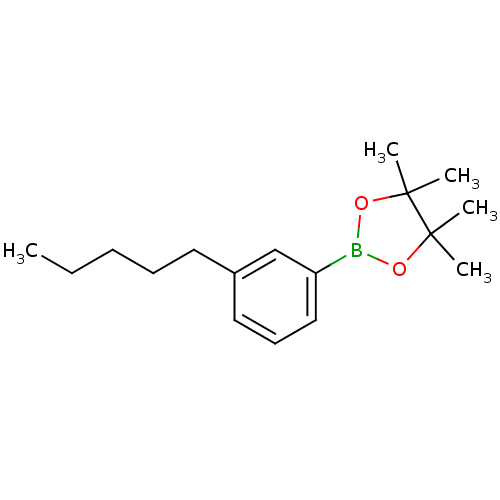 (CHEMBL1952306) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human lipoprotein lipase expressed using recombinant adenovirus using glycerol-tri[9,10(n)-3H]oleate after 1 hr by vesicle assay | Bioorg Med Chem Lett 22: 1397-401 (2012) Article DOI: 10.1016/j.bmcl.2011.12.043 BindingDB Entry DOI: 10.7270/Q28G8M5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444139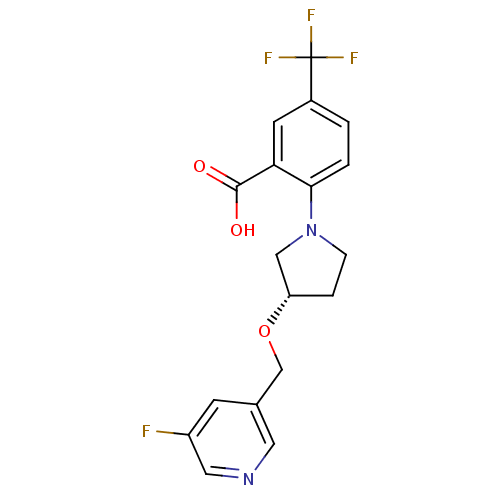 (CHEMBL3093395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoprotein lipase (Homo sapiens (Human)) | BDBM50444149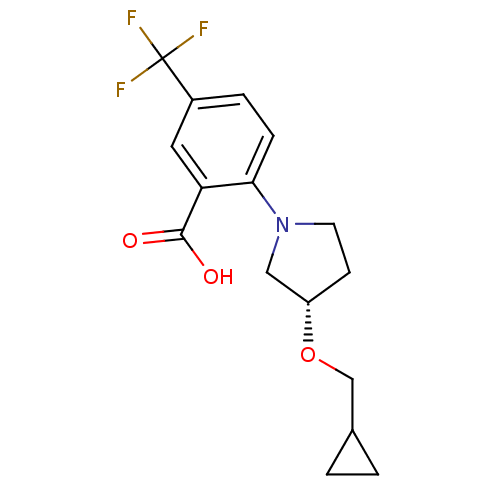 (CHEMBL3093308) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human lipoprotein lipase using bis-BODIPY-FL C11-PC as substrate preincubated for 30 mins followed by substrate addition by... | Bioorg Med Chem 21: 7724-34 (2013) Article DOI: 10.1016/j.bmc.2013.10.023 BindingDB Entry DOI: 10.7270/Q2P55PZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |