 Found 260 hits of ec50 for UniProtKB: P13631
Found 260 hits of ec50 for UniProtKB: P13631 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032225
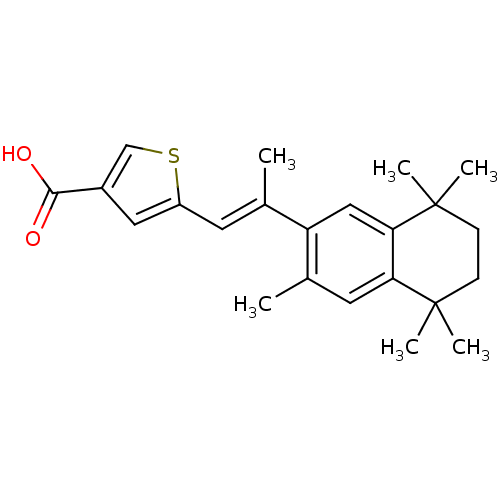
(5-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1cc(cs1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O2S/c1-14(9-17-11-16(13-26-17)21(24)25)18-12-20-19(10-15(18)2)22(3,4)7-8-23(20,5)6/h9-13H,7-8H2,1-6H3,(H,24,25)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032218
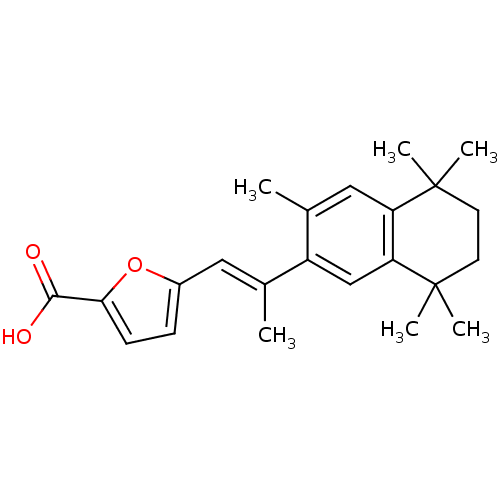
(5-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ccc(o1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O3/c1-14(11-16-7-8-20(26-16)21(24)25)17-13-19-18(12-15(17)2)22(3,4)9-10-23(19,5)6/h7-8,11-13H,9-10H2,1-6H3,(H,24,25)/b14-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032223

(4-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1csc(c1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O2S/c1-14(9-16-11-20(21(24)25)26-13-16)17-12-19-18(10-15(17)2)22(3,4)7-8-23(19,5)6/h9-13H,7-8H2,1-6H3,(H,24,25)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032224

(3-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1cccc(c1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H30O2/c1-16(12-18-8-7-9-19(14-18)23(26)27)20-15-22-21(13-17(20)2)24(3,4)10-11-25(22,5)6/h7-9,12-15H,10-11H2,1-6H3,(H,26,27)/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM323587
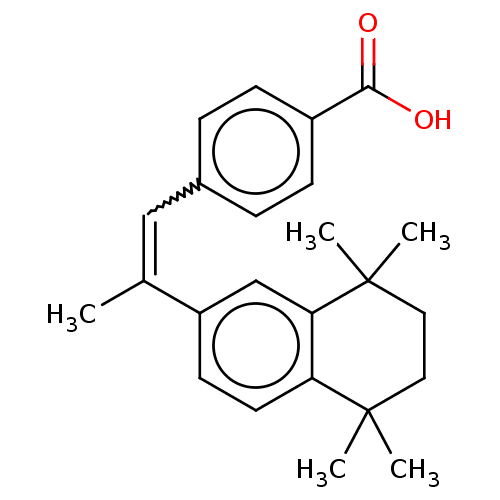
(4-[(E)-2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2...)Show SMILES CC(=Cc1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C |w:2.2| Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a |
ACUCELA INC.
US Patent
| Assay Description
Retinoid nuclear receptor activity is associated with transduction of the non-visual physiologic, pharmacologic, and toxicologic retinoid signals tha... |
US Patent US10188615 (2019)
BindingDB Entry DOI: 10.7270/Q2NP26HC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity at human RARgamma ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ... |
Bioorg Med Chem Lett 19: 489-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.040
BindingDB Entry DOI: 10.7270/Q2GF0TC9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457552

(CHEMBL4210595)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1cc(ccc1OCCCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C30H34O4/c1-29(2)14-15-30(3,4)26-19-23(10-12-25(26)29)24-18-22(11-13-27(24)34-17-5-16-31)20-6-8-21(9-7-20)28(32)33/h6-13,18-19,31H,5,14-17H2,1-4H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50282692

(4-[(E)-2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C23H26O2/c1-22(2)13-14-23(3,4)20-15-17(9-12-19(20)22)6-5-16-7-10-18(11-8-16)21(24)25/h5-12,15H,13-14H2,1-4H3,(H,24,25)/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR gamma receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50045276
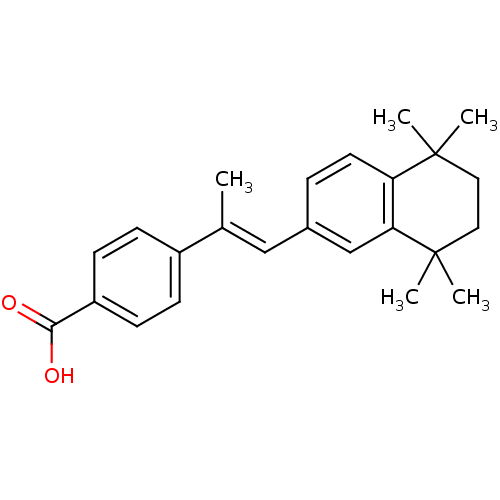
(4-[(E)-1-Methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tet...)Show SMILES C\C(=C/c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H28O2/c1-16(18-7-9-19(10-8-18)22(25)26)14-17-6-11-20-21(15-17)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR gamma receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR gamma receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation of retinoic acid receptor RAR gamma |
Bioorg Med Chem Lett 5: 523-527 (1995)
Article DOI: 10.1016/0960-894X(95)00065-2
BindingDB Entry DOI: 10.7270/Q20G3K4D |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 42: 4961-9 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PX3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM458152

(US10752616, Code No. BHBA-010)Show SMILES CC(C)c1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H18N2O4/c1-11(2)15-9-4-12(3)18-16(15)10-17(26-18)20-22-19(23-27-20)13-5-7-14(8-6-13)21(24)25/h4-11H,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50265920

(4-((5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24O2/c1-22(2)13-14-23(3,4)20-15-17(9-12-19(20)22)6-5-16-7-10-18(11-8-16)21(24)25/h7-12,15H,13-14H2,1-4H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity at human RARgamma ligand binding domain expressed in COS7 cells cotransfected with Gal4-DBD assessed as transcriptional activation after 16 ... |
Bioorg Med Chem Lett 19: 489-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.040
BindingDB Entry DOI: 10.7270/Q2GF0TC9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against retinoid X receptor using 5 nM of [3H]-9-cis-RA as a radioligand in baculovirus expressed receptor |
Bioorg Med Chem Lett 6: 213-218 (1996)
Article DOI: 10.1016/0960-894X(95)00588-K
BindingDB Entry DOI: 10.7270/Q2BC3ZHZ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing Retinoic acid receptor RAR gamma |
J Med Chem 38: 4764-7 (1996)
BindingDB Entry DOI: 10.7270/Q22N5194 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM323588
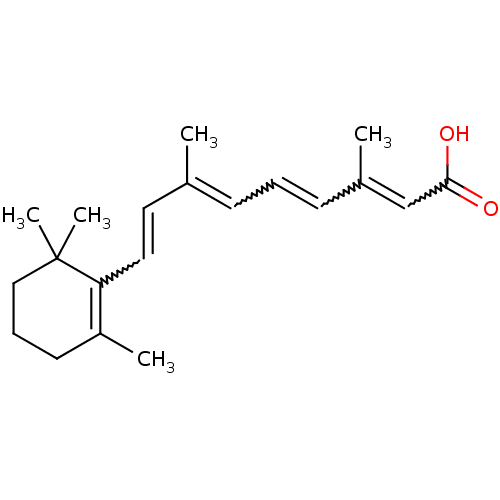
(Retinoic Acid | US10188615, at-RA | US10752616, Co...)Show SMILES CC(C=CC1=C(C)CCCC1(C)C)=CC=CC(C)=CC(O)=O |w:3.3,13.14,15.16,18.19,c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457563
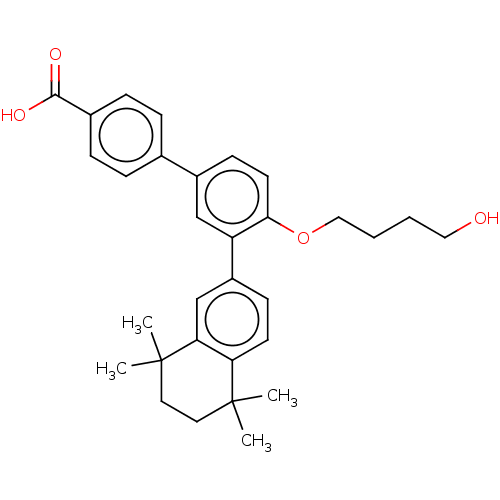
(CHEMBL4205733)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1cc(ccc1OCCCCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H36O4/c1-30(2)15-16-31(3,4)27-20-24(11-13-26(27)30)25-19-23(12-14-28(25)35-18-6-5-17-32)21-7-9-22(10-8-21)29(33)34/h7-14,19-20,32H,5-6,15-18H2,1-4H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50282691
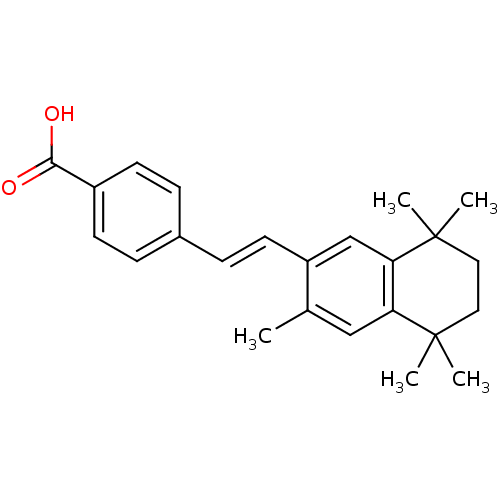
(4-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES Cc1cc2c(cc1\C=C\c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16-14-20-21(24(4,5)13-12-23(20,2)3)15-19(16)11-8-17-6-9-18(10-7-17)22(25)26/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR gamma receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457554

(CHEMBL4202830)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1cc(ccc1CCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C29H32O3/c1-28(2)14-15-29(3,4)26-18-23(11-12-25(26)28)24-17-22(10-7-20(24)13-16-30)19-5-8-21(9-6-19)27(31)32/h5-12,17-18,30H,13-16H2,1-4H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457560

(CHEMBL3939687)Show SMILES CCN(CC)c1ccc(cc1C(C)(C)C)-c1cc(ccc1OCCCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C30H37NO4/c1-6-31(7-2)27-15-13-24(20-26(27)30(3,4)5)25-19-23(14-16-28(25)35-18-8-17-32)21-9-11-22(12-10-21)29(33)34/h9-16,19-20,32H,6-8,17-18H2,1-5H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032219
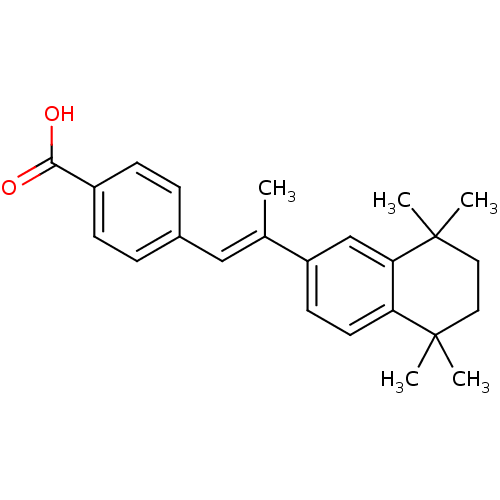
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against retinoic Acid gamma receptors cotransfected into CV-1 cells |
J Med Chem 37: 408-14 (1994)
BindingDB Entry DOI: 10.7270/Q20V8BT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity for retinoic acid receptor RAR gamma in transcriptional activation assay |
Bioorg Med Chem Lett 5: 2729-2734 (1995)
Article DOI: 10.1016/0960-894X(95)00455-3
BindingDB Entry DOI: 10.7270/Q22N527N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457546
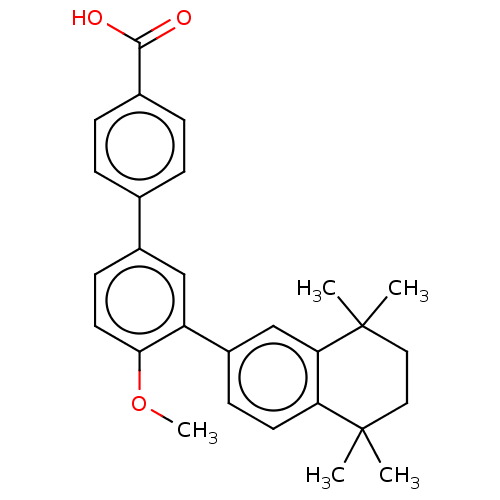
(CHEMBL4206237)Show SMILES COc1ccc(cc1-c1ccc2c(c1)C(C)(C)CCC2(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C28H30O3/c1-27(2)14-15-28(3,4)24-17-21(10-12-23(24)27)22-16-20(11-13-25(22)31-5)18-6-8-19(9-7-18)26(29)30/h6-13,16-17H,14-15H2,1-5H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457561

(CHEMBL3916016)Show SMILES CC(C)(C)c1cc(ccc1N1CCCC1)-c1cc(ccc1OCCCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C30H35NO4/c1-30(2,3)26-20-24(11-13-27(26)31-15-4-5-16-31)25-19-23(12-14-28(25)35-18-6-17-32)21-7-9-22(10-8-21)29(33)34/h7-14,19-20,32H,4-6,15-18H2,1-3H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM458284
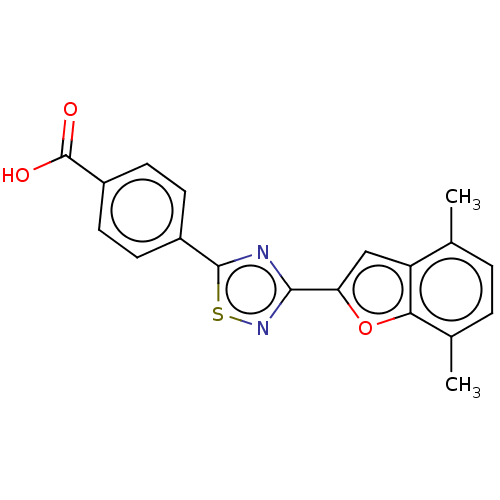
(US10752616, Code No. BHBA-042)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nsc(n1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O3S/c1-10-3-4-11(2)16-14(10)9-15(24-16)17-20-18(25-21-17)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation for RAR gamma receptor |
Bioorg Med Chem Lett 4: 1447-1452 (1994)
Article DOI: 10.1016/S0960-894X(01)80511-4
BindingDB Entry DOI: 10.7270/Q2B8582K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032219

((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation of retinoic acid receptor RAR gamma |
Bioorg Med Chem Lett 5: 523-527 (1995)
Article DOI: 10.1016/0960-894X(95)00065-2
BindingDB Entry DOI: 10.7270/Q20G3K4D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290184
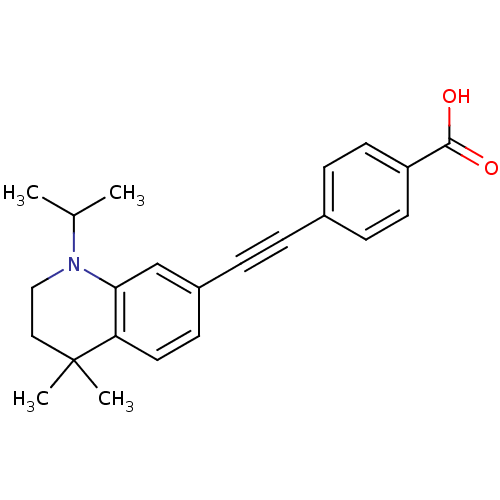
(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-12-9-18(15-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50048280

(6-(3-(1-Adamantyl)-4-methoxyphenyl)-2-naphthoic ac...)Show SMILES COc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc2cc(ccc2c1)C(O)=O |TLB:7:8:11:15.14.13,THB:9:10:13:17.8.16,9:8:11.10.15:13,16:8:11:15.14.13,16:14:11:17.9.8| Show InChI InChI=1S/C28H28O3/c1-31-26-7-6-23(21-2-3-22-12-24(27(29)30)5-4-20(22)11-21)13-25(26)28-14-17-8-18(15-28)10-19(9-17)16-28/h2-7,11-13,17-19H,8-10,14-16H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM458286

(US10752616, Code No. BHBA-044)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nsc(n1)-c1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C19H13FN2O3S/c1-9-3-4-10(2)16-13(9)8-15(25-16)17-21-18(26-22-17)11-5-6-12(19(23)24)14(20)7-11/h3-8H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457544

(CHEMBL3936922)Show SMILES CCN(CC)c1ccc(cc1C(C)(C)C)-c1cc(ccc1OCCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C29H35NO4/c1-6-30(7-2)26-14-12-23(19-25(26)29(3,4)5)24-18-22(13-15-27(24)34-17-16-31)20-8-10-21(11-9-20)28(32)33/h8-15,18-19,31H,6-7,16-17H2,1-5H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290179

(4-(1-Isopropyl-4,4-dimethyl-2-oxo-1,2,3,4-tetrahyd...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H23NO3/c1-15(2)24-20-12-9-17(13-19(20)23(3,4)14-21(24)25)6-5-16-7-10-18(11-8-16)22(26)27/h7-13,15H,14H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR gamma |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50097819
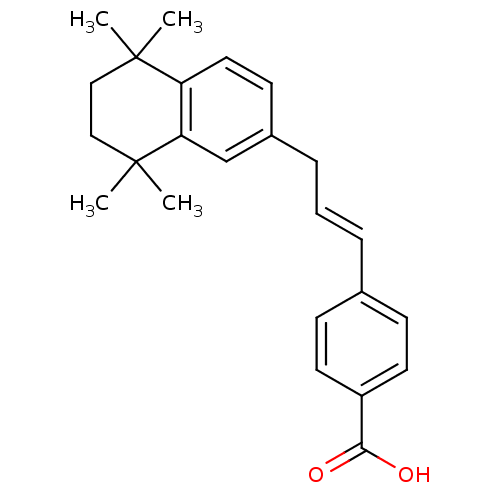
(4-[(E)-3-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(C\C=C\c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C24H28O2/c1-23(2)14-15-24(3,4)21-16-18(10-13-20(21)23)7-5-6-17-8-11-19(12-9-17)22(25)26/h5-6,8-13,16H,7,14-15H2,1-4H3,(H,25,26)/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards retinoic acid receptor beta was determined using [3H]-ATRA (5 nM) as radioligand |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457553

(CHEMBL4210099)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1cc(ccc1CO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C28H30O3/c1-27(2)13-14-28(3,4)25-16-21(11-12-24(25)27)23-15-20(9-10-22(23)17-29)18-5-7-19(8-6-18)26(30)31/h5-12,15-16,29H,13-14,17H2,1-4H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM458283
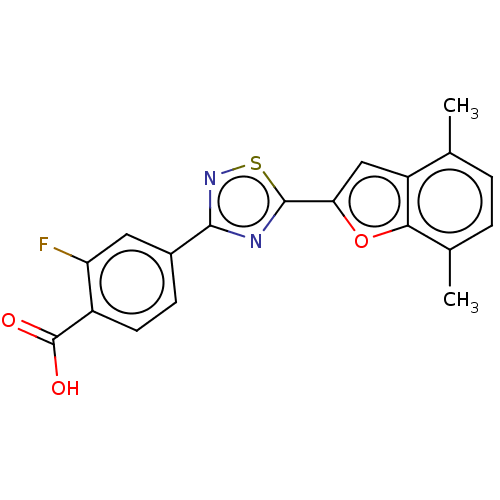
(US10752616, Code No. BHBA-041)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(ns1)-c1ccc(C(O)=O)c(F)c1 Show InChI InChI=1S/C19H13FN2O3S/c1-9-3-4-10(2)16-13(9)8-15(25-16)18-21-17(22-26-18)11-5-6-12(19(23)24)14(20)7-11/h3-8H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457549
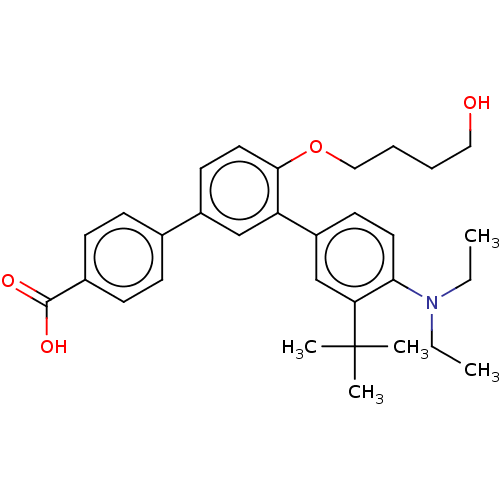
(CHEMBL3960035)Show SMILES CCN(CC)c1ccc(cc1C(C)(C)C)-c1cc(ccc1OCCCCO)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H39NO4/c1-6-32(7-2)28-16-14-25(21-27(28)31(3,4)5)26-20-24(15-17-29(26)36-19-9-8-18-33)22-10-12-23(13-11-22)30(34)35/h10-17,20-21,33H,6-9,18-19H2,1-5H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50526315
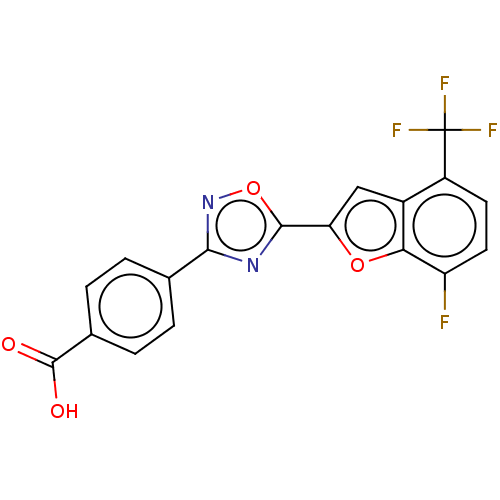
(CHEMBL4439399 | US10752616, Code No. BHBA-019)Show SMILES OC(=O)c1ccc(cc1)-c1noc(n1)-c1cc2c(ccc(F)c2o1)C(F)(F)F Show InChI InChI=1S/C18H8F4N2O4/c19-12-6-5-11(18(20,21)22)10-7-13(27-14(10)12)16-23-15(24-28-16)8-1-3-9(4-2-8)17(25)26/h1-7H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Allergan Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards retinoic acid receptor gamma was determined using [3H]-ATRA (5 nM) as radioligand |
Bioorg Med Chem Lett 11: 765-8 (2001)
BindingDB Entry DOI: 10.7270/Q2348JN3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoic acid receptor RAR gamma |
J Med Chem 42: 4961-9 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PX3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Effective concentration for retinoic acid receptor RAR alpha transcriptional activation |
Bioorg Med Chem Lett 12: 3145-8 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7H0H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50457551
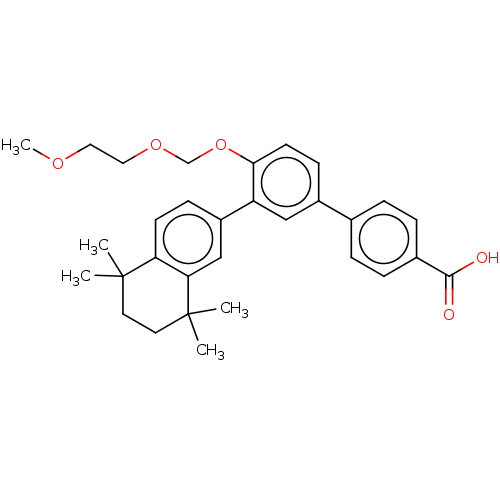
(CHEMBL4217953)Show SMILES COCCOCOc1ccc(cc1-c1ccc2c(c1)C(C)(C)CCC2(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C31H36O5/c1-30(2)14-15-31(3,4)27-19-24(10-12-26(27)30)25-18-23(21-6-8-22(9-7-21)29(32)33)11-13-28(25)36-20-35-17-16-34-5/h6-13,18-19H,14-17,20H2,1-5H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain-tagged RARgamma (unknown origin) ligand-binding domain expressed in human HG5LN cells incubated for 18 hr... |
Bioorg Med Chem Lett 28: 1736-1741 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.036
BindingDB Entry DOI: 10.7270/Q2N300JQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM458251

(US10752616, Code No. BHBA-016)Show SMILES Cc1ccc(Cl)c2cc(oc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H11ClN2O4/c1-9-2-7-13(19)12-8-14(24-15(9)12)17-20-16(21-25-17)10-3-5-11(6-4-10)18(22)23/h2-8H,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
BindingDB Entry DOI: 10.7270/Q2B85C6M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290185

(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-15-18(9-12-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity RARgamma (unknown origin) assessed as induction of receptor transactivation |
J Med Chem 62: 2112-2126 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01848
BindingDB Entry DOI: 10.7270/Q2J38X18 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity RARgamma (unknown origin) assessed as induction of receptor transactivation |
J Med Chem 62: 2112-2126 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01848
BindingDB Entry DOI: 10.7270/Q2J38X18 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data