

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay | J Med Chem 37: 408-14 (1994) BindingDB Entry DOI: 10.7270/Q20V8BT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50218464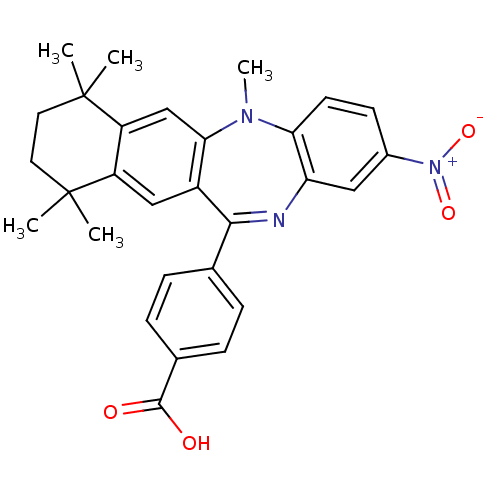 ((Z)-4-(5,7,7,10,10-pentamethyl-2-nitro-7,8,9,10-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50044098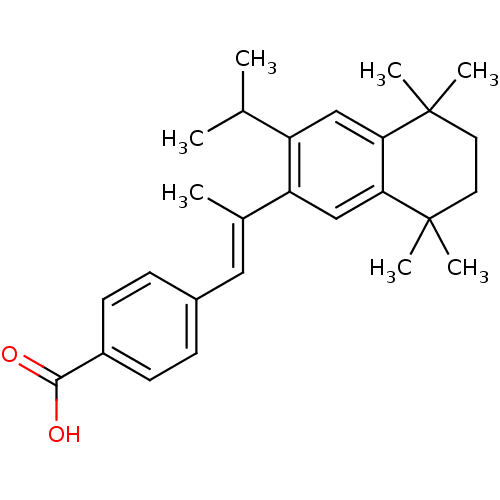 (4-[(E)-2-(3-Isopropyl-5,5,8,8-tetramethyl-5,6,7,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay | J Med Chem 37: 408-14 (1994) BindingDB Entry DOI: 10.7270/Q20V8BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50044099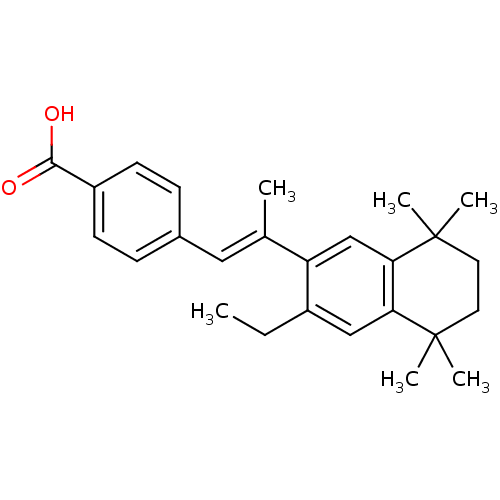 (4-[(E)-2-(3-Ethyl-5,5,8,8-tetramethyl-5,6,7,8-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay | J Med Chem 37: 408-14 (1994) BindingDB Entry DOI: 10.7270/Q20V8BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524173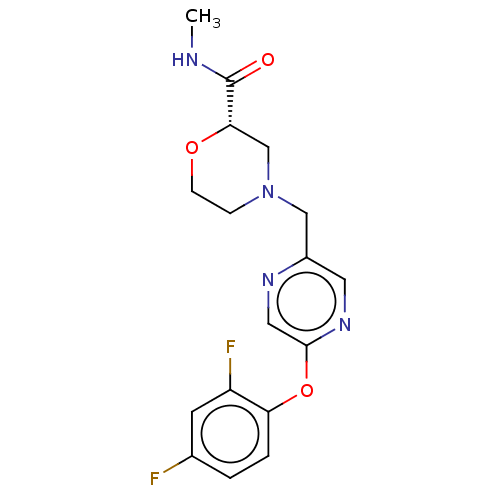 (US11166958, Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524172 (US11166958, Example 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524169 (US11166958, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524182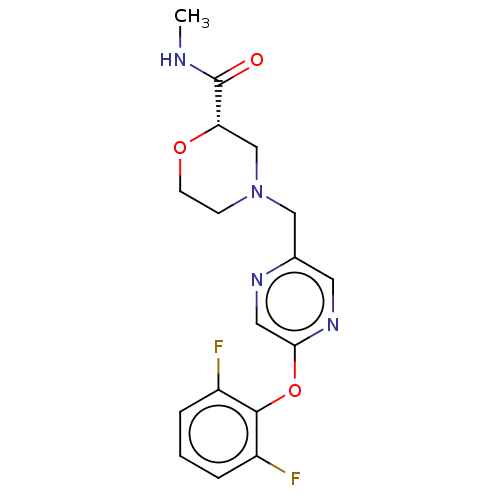 (US11166958, Example 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524176 (US11166958, Example 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524177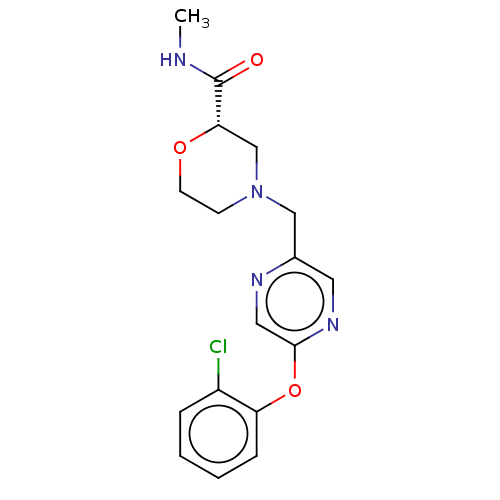 (US11166958, Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524179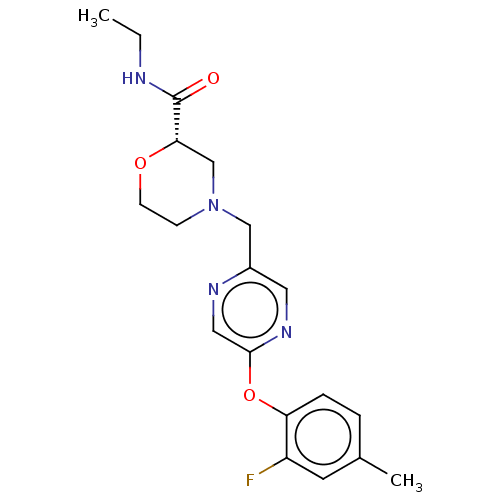 (US11166958, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524183 (US11166958, Example 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 542 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524178 (US11166958, Example 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 595 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524181 (US11166958, Example 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 644 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524170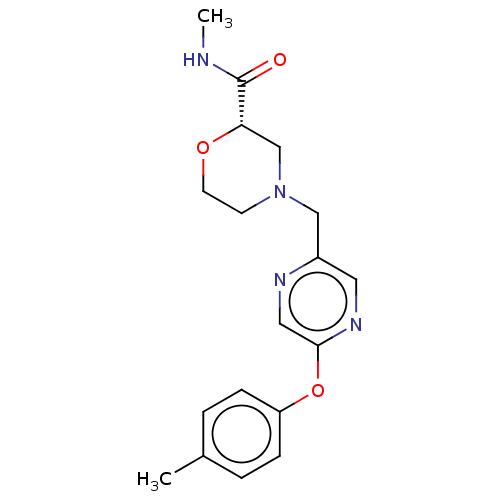 (US11166958, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256177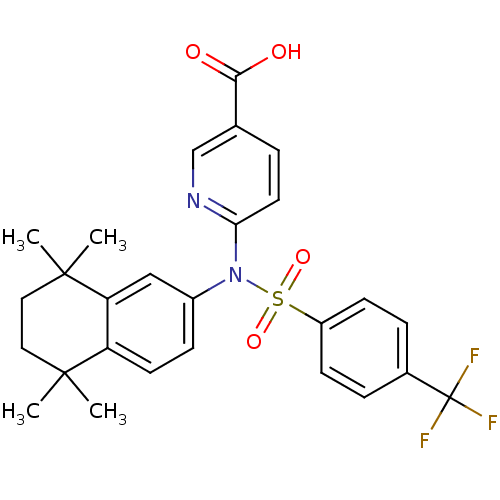 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524180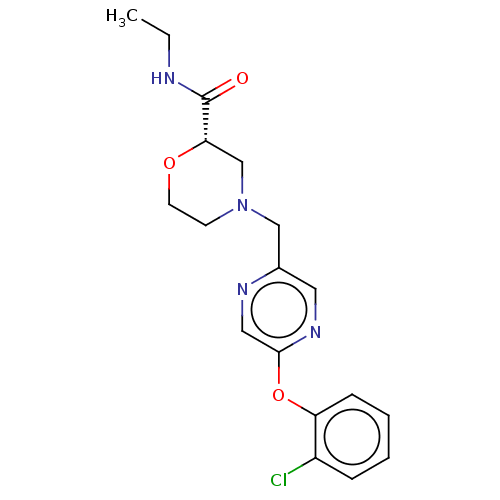 (US11166958, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 807 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524175 (US11166958, Example 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 856 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256176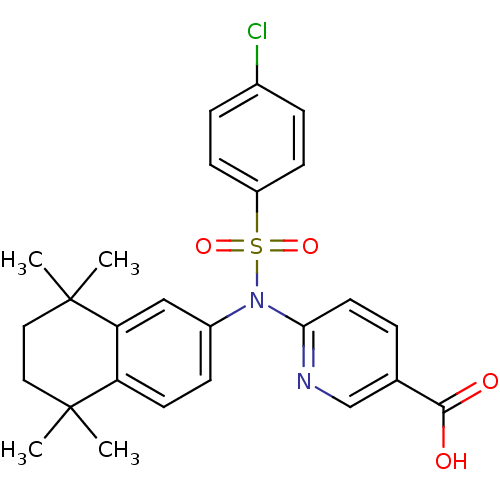 (6-(4-chloro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524168 (US11166958, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 968 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50032219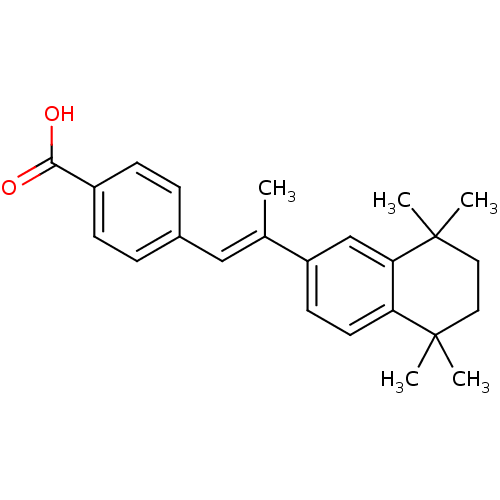 ((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay | J Med Chem 37: 408-14 (1994) BindingDB Entry DOI: 10.7270/Q20V8BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50032221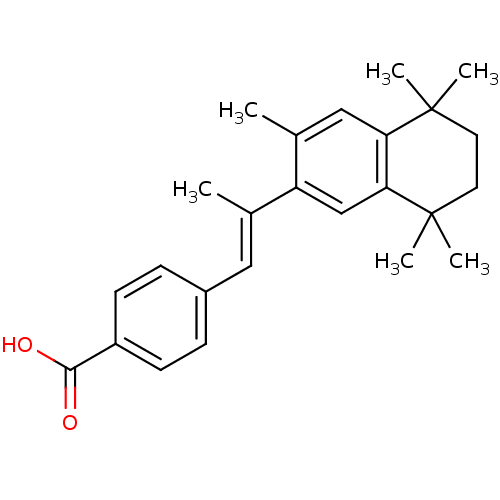 (4-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay | J Med Chem 37: 408-14 (1994) BindingDB Entry DOI: 10.7270/Q20V8BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assay | J Med Chem 37: 408-14 (1994) BindingDB Entry DOI: 10.7270/Q20V8BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524174 (US11166958, Example 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524171 (US11166958, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256174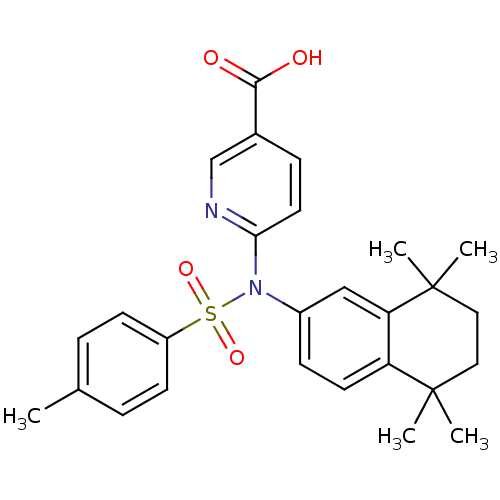 (6-(4-methyl-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256175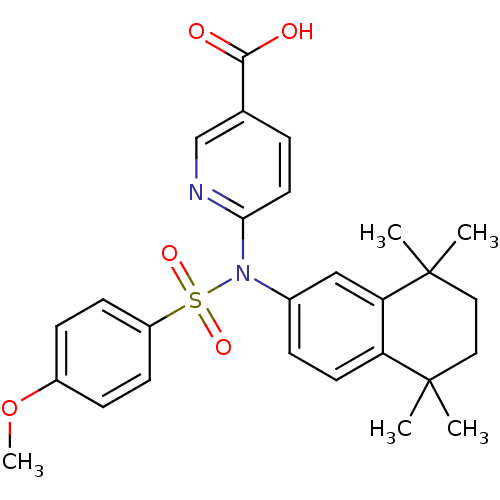 (6-(4-methoxy-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524184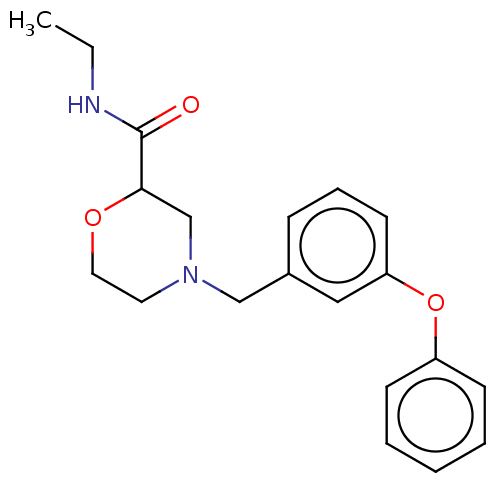 (WO2015/130905, Example 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524186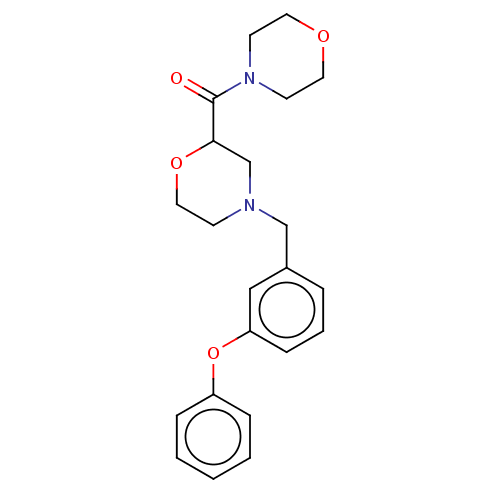 (WO2015/130905, Example 106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524187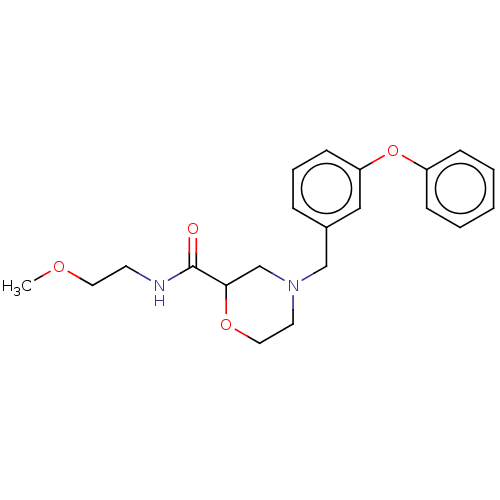 (WO2015/130905, Example 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM524185 (WO2015/130905, Example 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description At the assay day cells were washed 3× with assay buffer (as described above), 10 μL buffer remained in the wells after washing. 10 μL Ca ki... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KW5K6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50454190 (CHEMBL4202767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-retinoic acid from recombinant human full length RXRbeta after 24 hrs by scintillation counting analysis | Bioorg Med Chem 26: 798-814 (2018) Article DOI: 10.1016/j.bmc.2017.12.015 BindingDB Entry DOI: 10.7270/Q2WH2SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256275 (6-(4-nitro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256123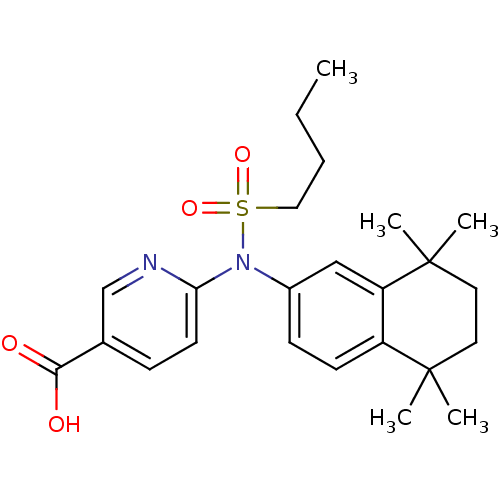 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256124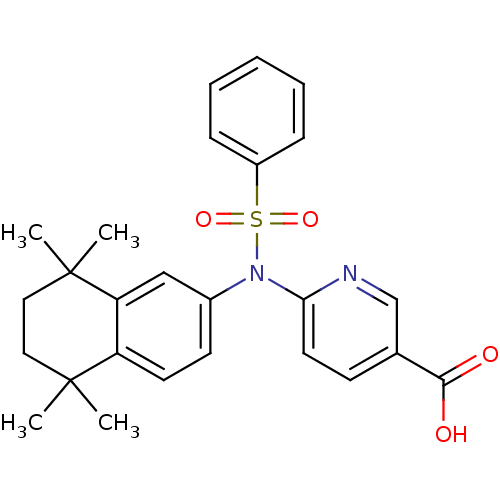 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50276797 (CHEMBL4174856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at recombinant GST-tagged human RXRbeta LBD assessed as inhibition of all-trans-retinoic acid-induced fluorescein-labeled coactiv... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||