 Found 371 hits of ic50 data for polymerid = 50000279
Found 371 hits of ic50 data for polymerid = 50000279 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356075
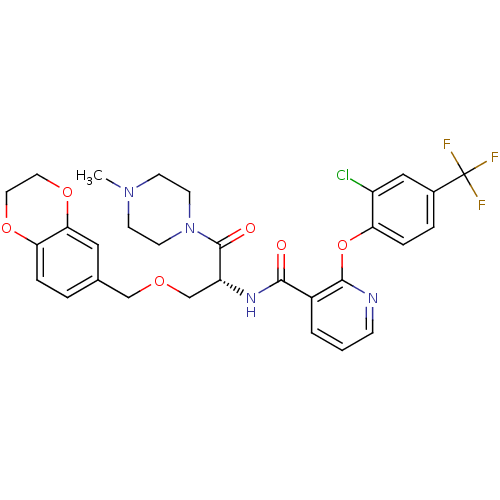
(CHEMBL1911818)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccc2OCCOc2c1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C30H30ClF3N4O6/c1-37-9-11-38(12-10-37)29(40)23(18-41-17-19-4-6-25-26(15-19)43-14-13-42-25)36-27(39)21-3-2-8-35-28(21)44-24-7-5-20(16-22(24)31)30(32,33)34/h2-8,15-16,23H,9-14,17-18H2,1H3,(H,36,39)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339792
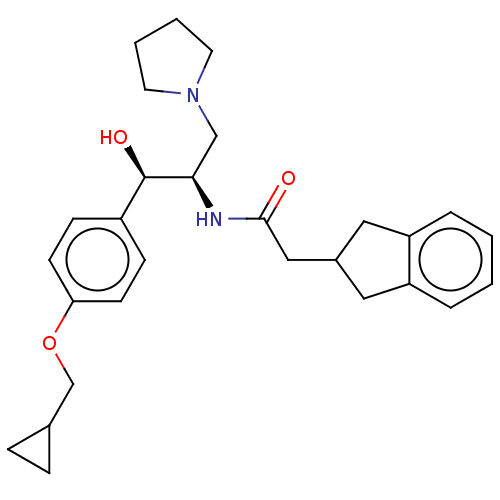
(US10202340, Example 11)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1)c1ccc(OCC2CC2)cc1 |r| Show InChI InChI=1S/C28H36N2O3/c31-27(17-21-15-23-5-1-2-6-24(23)16-21)29-26(18-30-13-3-4-14-30)28(32)22-9-11-25(12-10-22)33-19-20-7-8-20/h1-2,5-6,9-12,20-21,26,28,32H,3-4,7-8,13-19H2,(H,29,31)/t26-,28-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
)Inhibition of GlcCer production in MDCKII cells stably expressing human MDR1 (obtained from The Netherlands Cancer Institute). |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339793

(US10202340, Example 12)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1)c1ccc(OCC(F)(F)F)cc1 |r| Show InChI InChI=1S/C26H31F3N2O3/c27-26(28,29)17-34-22-9-7-19(8-10-22)25(33)23(16-31-11-3-4-12-31)30-24(32)15-18-13-20-5-1-2-6-21(20)14-18/h1-2,5-10,18,23,25,33H,3-4,11-17H2,(H,30,32)/t23-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5290844
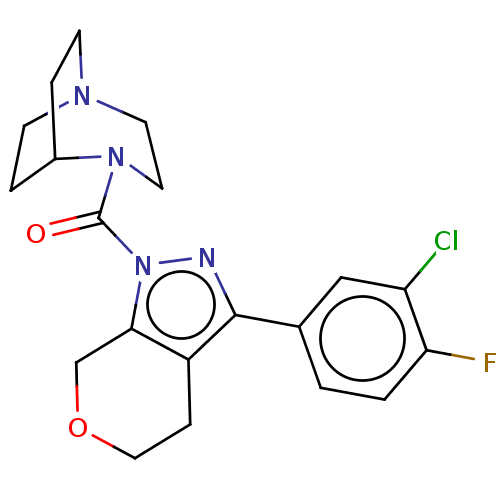
Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356075

(CHEMBL1911818)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccc2OCCOc2c1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C30H30ClF3N4O6/c1-37-9-11-38(12-10-37)29(40)23(18-41-17-19-4-6-25-26(15-19)43-14-13-42-25)36-27(39)21-3-2-8-35-28(21)44-24-7-5-20(16-22(24)31)30(32,33)34/h2-8,15-16,23H,9-14,17-18H2,1H3,(H,36,39)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356076
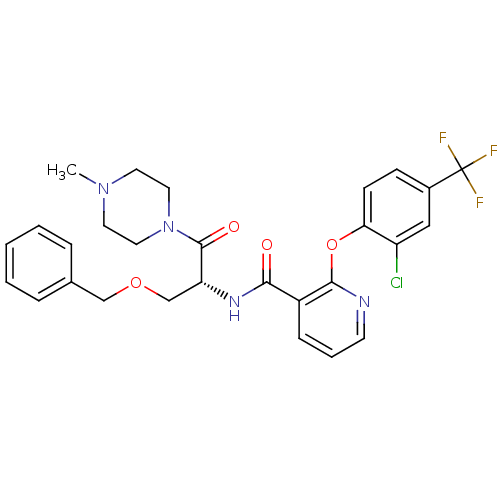
(CHEMBL1911817)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-35-12-14-36(15-13-35)27(38)23(18-39-17-19-6-3-2-4-7-19)34-25(37)21-8-5-11-33-26(21)40-24-10-9-20(16-22(24)29)28(30,31)32/h2-11,16,23H,12-15,17-18H2,1H3,(H,34,37)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339792

(US10202340, Example 11)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1)c1ccc(OCC2CC2)cc1 |r| Show InChI InChI=1S/C28H36N2O3/c31-27(17-21-15-23-5-1-2-6-24(23)16-21)29-26(18-30-13-3-4-14-30)28(32)22-9-11-25(12-10-22)33-19-20-7-8-20/h1-2,5-6,9-12,20-21,26,28,32H,3-4,7-8,13-19H2,(H,29,31)/t26-,28-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | CHEMBL5275403

Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356077
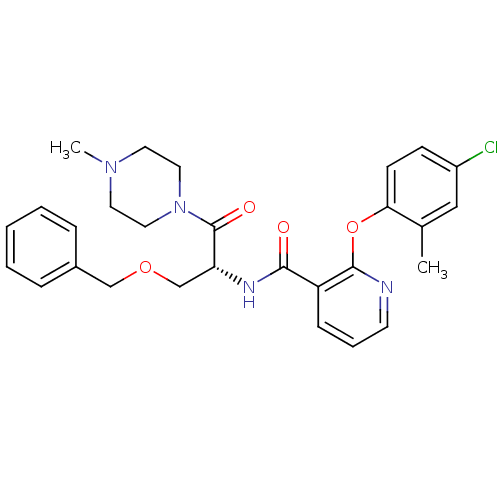
(CHEMBL1911816)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(Cl)cc1C |r| Show InChI InChI=1S/C28H31ClN4O4/c1-20-17-22(29)10-11-25(20)37-27-23(9-6-12-30-27)26(34)31-24(19-36-18-21-7-4-3-5-8-21)28(35)33-15-13-32(2)14-16-33/h3-12,17,24H,13-16,18-19H2,1-2H3,(H,31,34)/t24-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356078
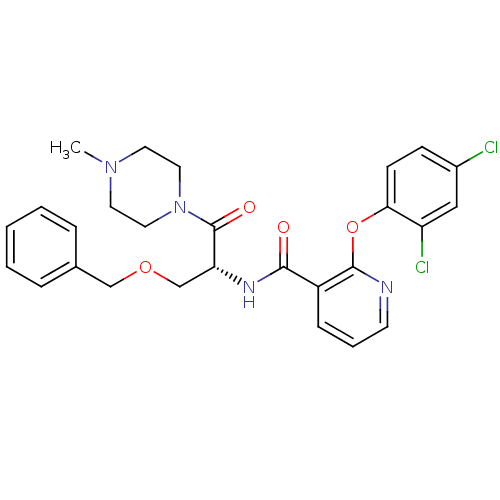
(CHEMBL1911815)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C27H28Cl2N4O4/c1-32-12-14-33(15-13-32)27(35)23(18-36-17-19-6-3-2-4-7-19)31-25(34)21-8-5-11-30-26(21)37-24-10-9-20(28)16-22(24)29/h2-11,16,23H,12-15,17-18H2,1H3,(H,31,34)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356076

(CHEMBL1911817)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-35-12-14-36(15-13-35)27(38)23(18-39-17-19-6-3-2-4-7-19)34-25(37)21-8-5-11-33-26(21)40-24-10-9-20(16-22(24)29)28(30,31)32/h2-11,16,23H,12-15,17-18H2,1H3,(H,34,37)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091
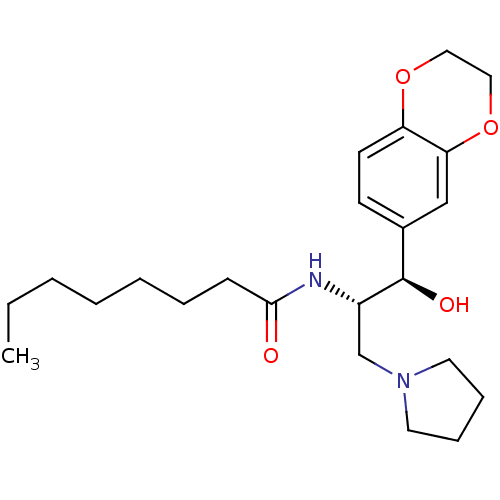
(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090
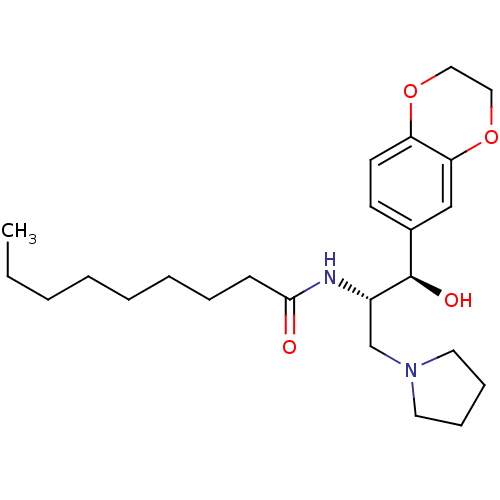
(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394804
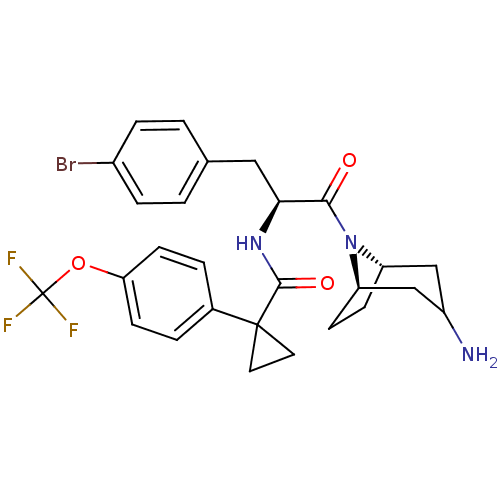
(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090

(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807
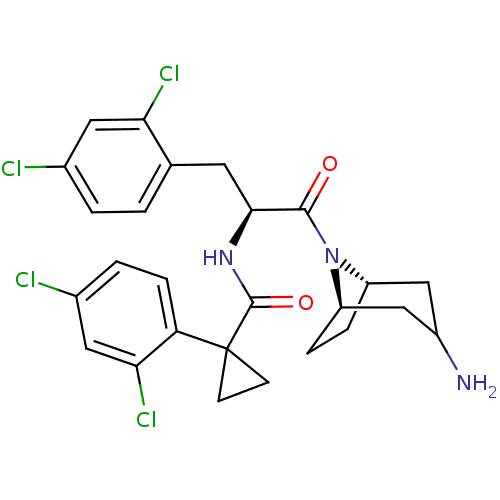
(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091

(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820
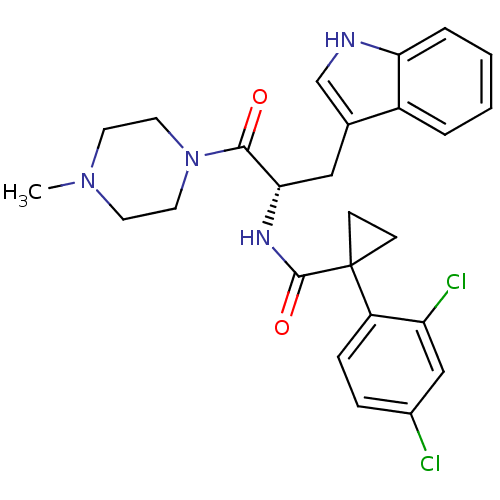
(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394812
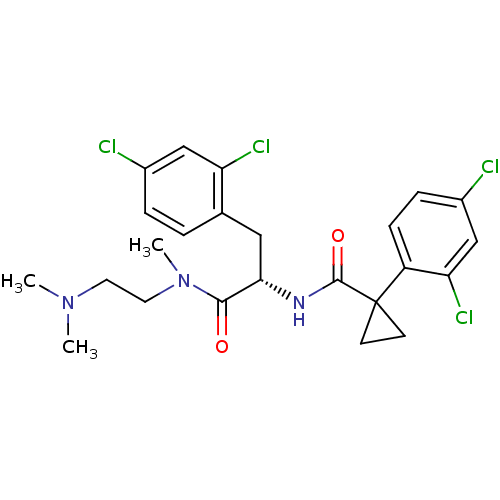
(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028257
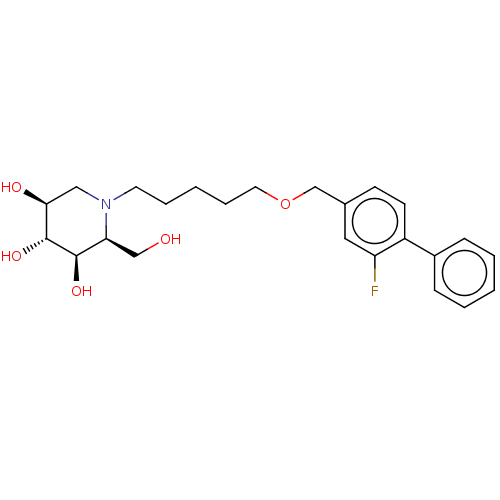
(CHEMBL3354637)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(c(F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C24H32FNO5/c25-20-13-17(9-10-19(20)18-7-3-1-4-8-18)16-31-12-6-2-5-11-26-14-22(28)24(30)23(29)21(26)15-27/h1,3-4,7-10,13,21-24,27-30H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339793

(US10202340, Example 12)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1)c1ccc(OCC(F)(F)F)cc1 |r| Show InChI InChI=1S/C26H31F3N2O3/c27-26(28,29)17-34-22-9-7-19(8-10-22)25(33)23(16-31-11-3-4-12-31)30-24(32)15-18-13-20-5-1-2-6-21(20)14-18/h1-2,5-10,18,23,25,33H,3-4,11-17H2,(H,30,32)/t23-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
)Inhibition of GlcCer production in MDCKII cells stably expressing human MDR1 (obtained from The Netherlands Cancer Institute). |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356079
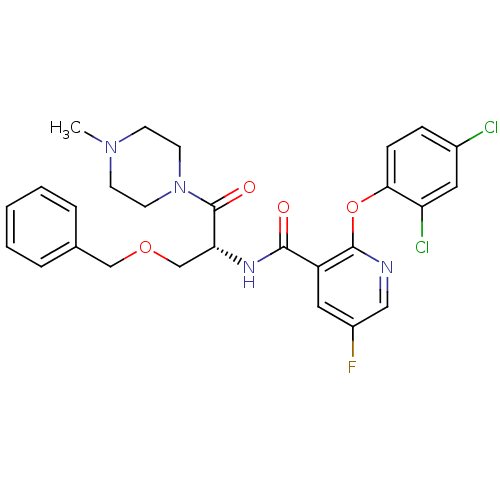
(CHEMBL1911814)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cc(F)cnc1Oc1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C27H27Cl2FN4O4/c1-33-9-11-34(12-10-33)27(36)23(17-37-16-18-5-3-2-4-6-18)32-25(35)21-14-20(30)15-31-26(21)38-24-8-7-19(28)13-22(24)29/h2-8,13-15,23H,9-12,16-17H2,1H3,(H,32,35)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028217
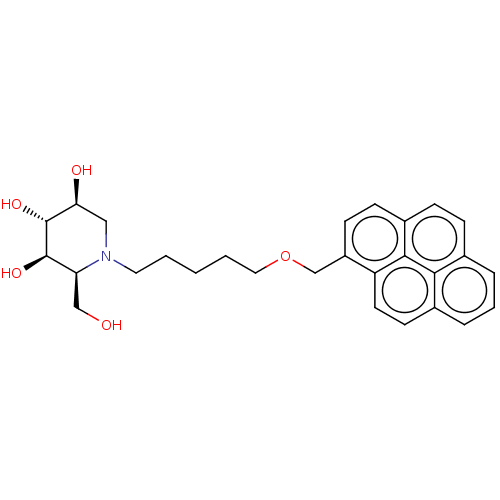
(CHEMBL3354037)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccc3cccc4ccc1c2c34 |r| Show InChI InChI=1S/C28H33NO5/c30-16-23-27(32)28(33)24(31)15-29(23)13-2-1-3-14-34-17-21-10-9-20-8-7-18-5-4-6-19-11-12-22(21)26(20)25(18)19/h4-12,23-24,27-28,30-33H,1-3,13-17H2/t23-,24-,27+,28+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028215
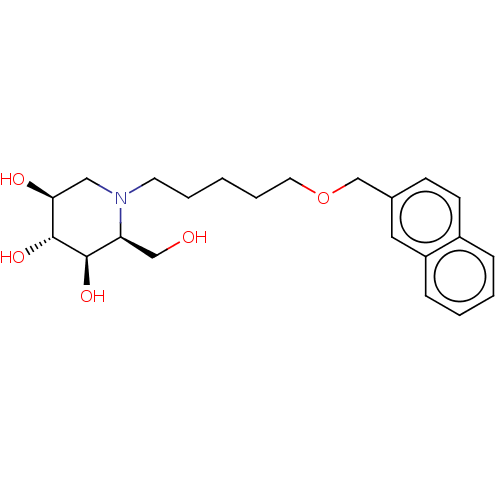
(CHEMBL3354035)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H31NO5/c24-14-19-21(26)22(27)20(25)13-23(19)10-4-1-5-11-28-15-16-8-9-17-6-2-3-7-18(17)12-16/h2-3,6-9,12,19-22,24-27H,1,4-5,10-11,13-15H2/t19-,20-,21+,22+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028206
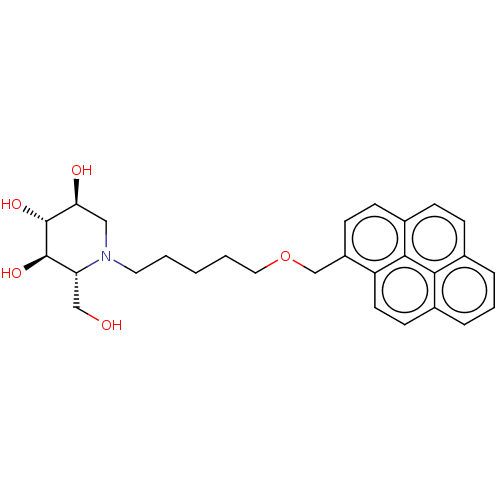
(CHEMBL3354024)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccc3cccc4ccc1c2c34 |r| Show InChI InChI=1S/C28H33NO5/c30-16-23-27(32)28(33)24(31)15-29(23)13-2-1-3-14-34-17-21-10-9-20-8-7-18-5-4-6-19-11-12-22(21)26(20)25(18)19/h4-12,23-24,27-28,30-33H,1-3,13-17H2/t23-,24+,27-,28-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028204
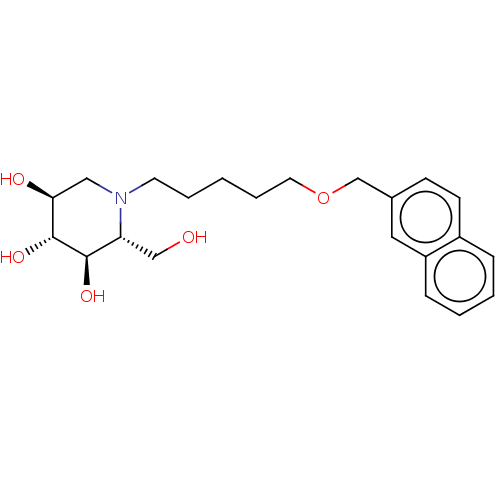
(CHEMBL3354022)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H31NO5/c24-14-19-21(26)22(27)20(25)13-23(19)10-4-1-5-11-28-15-16-8-9-17-6-2-3-7-18(17)12-16/h2-3,6-9,12,19-22,24-27H,1,4-5,10-11,13-15H2/t19-,20+,21-,22-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028255
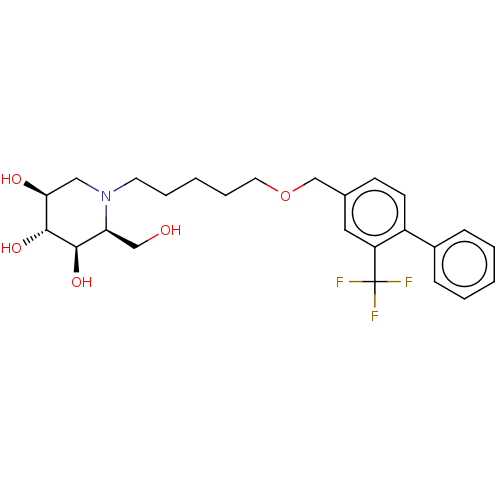
(CHEMBL3354635)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C25H32F3NO5/c26-25(27,28)20-13-17(9-10-19(20)18-7-3-1-4-8-18)16-34-12-6-2-5-11-29-14-22(31)24(33)23(32)21(29)15-30/h1,3-4,7-10,13,21-24,30-33H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028254
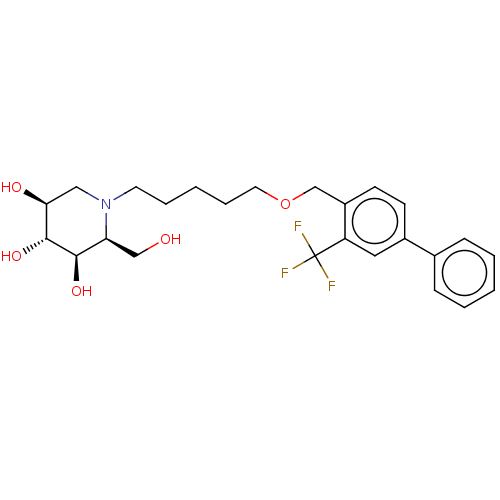
(CHEMBL3354634)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(cc1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C25H32F3NO5/c26-25(27,28)20-13-18(17-7-3-1-4-8-17)9-10-19(20)16-34-12-6-2-5-11-29-14-22(31)24(33)23(32)21(29)15-30/h1,3-4,7-10,13,21-24,30-33H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339782
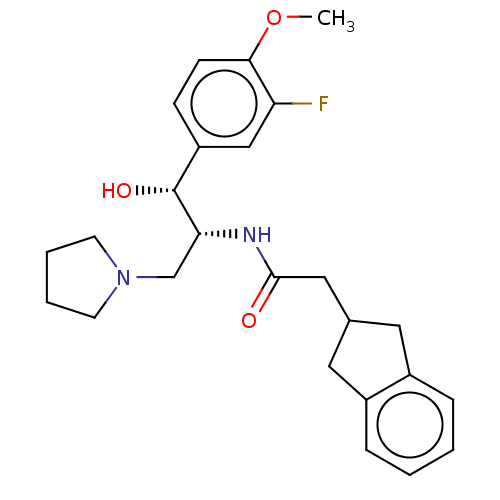
(US10202340, Example 1)Show SMILES COc1ccc(cc1F)[C@@H](O)[C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1 |r| Show InChI InChI=1S/C25H31FN2O3/c1-31-23-9-8-20(15-21(23)26)25(30)22(16-28-10-4-5-11-28)27-24(29)14-17-12-18-6-2-3-7-19(18)13-17/h2-3,6-9,15,17,22,25,30H,4-5,10-14,16H2,1H3,(H,27,29)/t22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339783
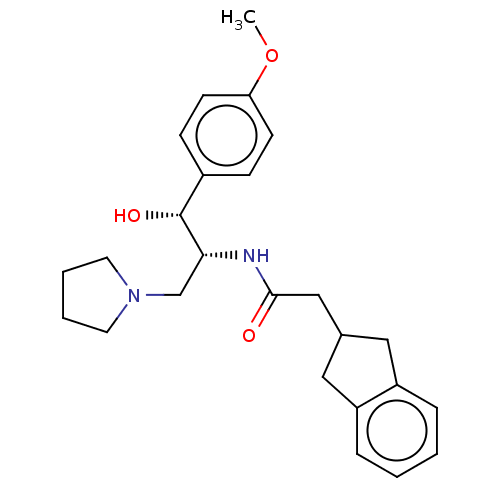
(US10202340, Example 2)Show SMILES COc1ccc(cc1)[C@@H](O)[C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1 |r| Show InChI InChI=1S/C25H32N2O3/c1-30-22-10-8-19(9-11-22)25(29)23(17-27-12-4-5-13-27)26-24(28)16-18-14-20-6-2-3-7-21(20)15-18/h2-3,6-11,18,23,25,29H,4-5,12-17H2,1H3,(H,26,28)/t23-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394809
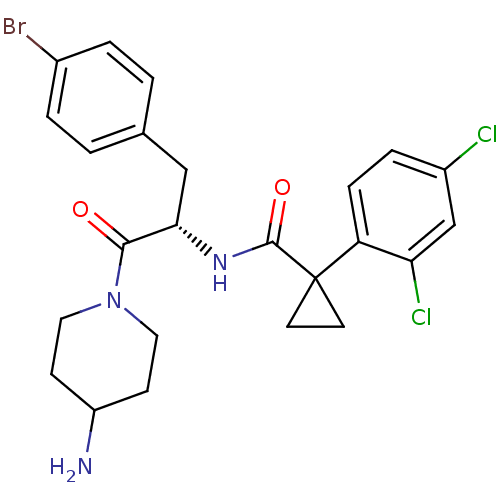
(CHEMBL2163823)Show SMILES NC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c25-16-3-1-15(2-4-16)13-21(22(31)30-11-7-18(28)8-12-30)29-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21H,7-13,28H2,(H,29,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394805
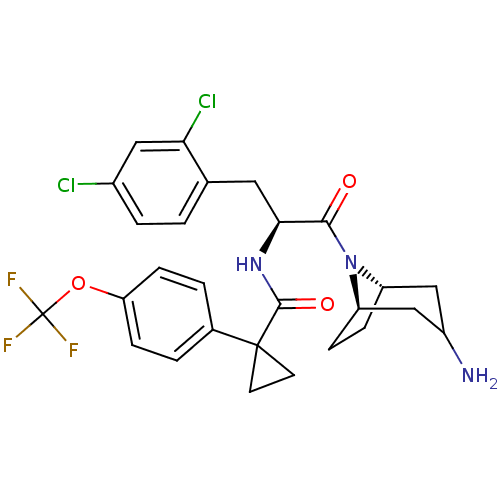
(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394816
(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50602025
(CHEMBL5181454)Show SMILES CC(C)(O)c1ccc(cc1)N1Cc2c(cc(nc2-c2ccccc2OCC(F)(F)F)C2(C)COC2)C1=O | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129039
BindingDB Entry DOI: 10.7270/Q2ZW1R16 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339788
(US10202340, Example 7)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C25H29F3N2O3/c26-25(27,28)33-21-9-7-18(8-10-21)24(32)22(16-30-11-3-4-12-30)29-23(31)15-17-13-19-5-1-2-6-20(19)14-17/h1-2,5-10,17,22,24,32H,3-4,11-16H2,(H,29,31)/t22-,24-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394811
(CHEMBL2163839)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H28BrCl2N3O2/c1-29(2)12-13-30(3)22(31)21(14-16-4-6-17(25)7-5-16)28-23(32)24(10-11-24)19-9-8-18(26)15-20(19)27/h4-9,15,21H,10-14H2,1-3H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356094

(CHEMBL1911829)Show SMILES FC(F)(F)c1ccc(Oc2ncccc2C(=O)N[C@H](COCc2ccccc2)C(=O)N[C@@H]2CN3CCC2CC3)c(Cl)c1 |r,wD:18.18,31.32,(8.76,-41.16,;8.77,-39.62,;10.11,-38.85,;10.1,-40.39,;7.44,-38.85,;6.1,-39.61,;4.77,-38.83,;4.79,-37.3,;3.46,-36.52,;3.47,-34.98,;4.8,-34.21,;4.8,-32.67,;3.46,-31.9,;2.13,-32.67,;2.14,-34.2,;.8,-34.97,;.8,-36.51,;-.53,-34.2,;-1.86,-34.96,;-1.87,-36.5,;-3.2,-37.27,;-3.2,-38.81,;-1.87,-39.59,;-1.87,-41.13,;-.54,-41.91,;.8,-41.14,;.79,-39.59,;-.54,-38.83,;-3.2,-34.19,;-3.19,-32.65,;-4.53,-34.96,;-5.86,-34.19,;-7.2,-34.96,;-8.53,-34.18,;-8.53,-32.64,;-7.19,-31.88,;-5.86,-32.65,;-7.4,-32.68,;-7,-34.18,;6.11,-36.53,;6.11,-34.99,;7.44,-37.3,)| Show InChI InChI=1S/C30H30ClF3N4O4/c31-23-15-21(30(32,33)34)8-9-26(23)42-29-22(7-4-12-35-29)27(39)37-25(18-41-17-19-5-2-1-3-6-19)28(40)36-24-16-38-13-10-20(24)11-14-38/h1-9,12,15,20,24-25H,10-11,13-14,16-18H2,(H,36,40)(H,37,39)/t24-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394817
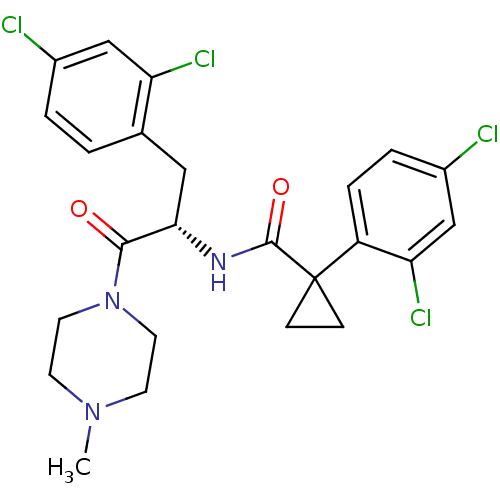
(CHEMBL2163832)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-30-8-10-31(11-9-30)22(32)21(12-15-2-3-16(25)13-19(15)27)29-23(33)24(6-7-24)18-5-4-17(26)14-20(18)28/h2-5,13-14,21H,6-12H2,1H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807

(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50602022
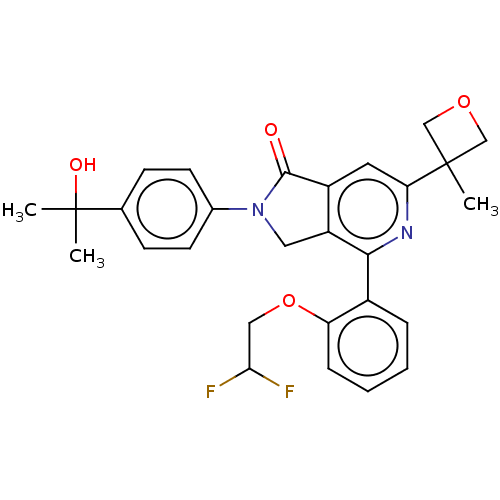
(CHEMBL5197585)Show SMILES CC(C)(O)c1ccc(cc1)N1Cc2c(cc(nc2-c2ccccc2OCC(F)F)C2(C)COC2)C1=O | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129039
BindingDB Entry DOI: 10.7270/Q2ZW1R16 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356066
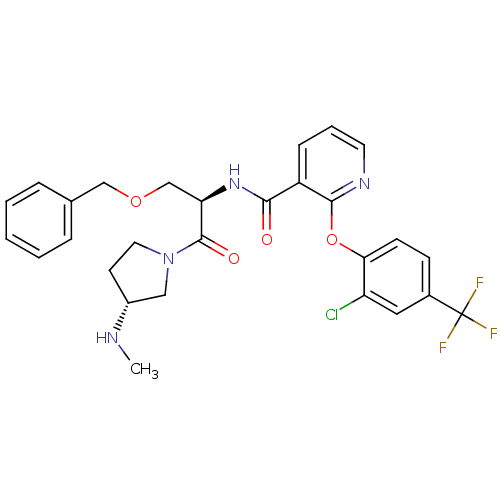
(CHEMBL1911827)Show SMILES CN[C@@H]1CCN(C1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-33-20-11-13-36(15-20)27(38)23(17-39-16-18-6-3-2-4-7-18)35-25(37)21-8-5-12-34-26(21)40-24-10-9-19(14-22(24)29)28(30,31)32/h2-10,12,14,20,23,33H,11,13,15-17H2,1H3,(H,35,37)/t20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339785
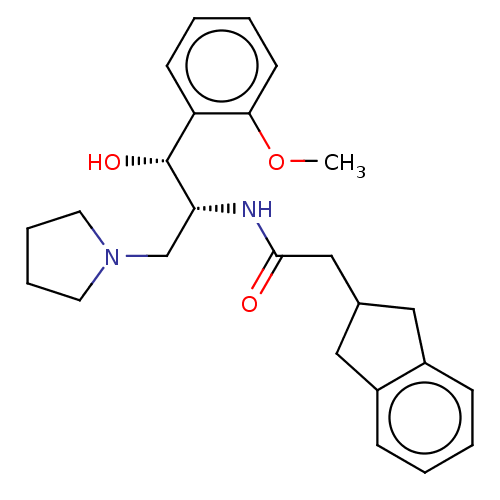
(US10202340, Example 4)Show SMILES COc1ccccc1[C@@H](O)[C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1 |r| Show InChI InChI=1S/C25H32N2O3/c1-30-23-11-5-4-10-21(23)25(29)22(17-27-12-6-7-13-27)26-24(28)16-18-14-19-8-2-3-9-20(19)15-18/h2-5,8-11,18,22,25,29H,6-7,12-17H2,1H3,(H,26,28)/t22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356094

(CHEMBL1911829)Show SMILES FC(F)(F)c1ccc(Oc2ncccc2C(=O)N[C@H](COCc2ccccc2)C(=O)N[C@@H]2CN3CCC2CC3)c(Cl)c1 |r,wD:18.18,31.32,(8.76,-41.16,;8.77,-39.62,;10.11,-38.85,;10.1,-40.39,;7.44,-38.85,;6.1,-39.61,;4.77,-38.83,;4.79,-37.3,;3.46,-36.52,;3.47,-34.98,;4.8,-34.21,;4.8,-32.67,;3.46,-31.9,;2.13,-32.67,;2.14,-34.2,;.8,-34.97,;.8,-36.51,;-.53,-34.2,;-1.86,-34.96,;-1.87,-36.5,;-3.2,-37.27,;-3.2,-38.81,;-1.87,-39.59,;-1.87,-41.13,;-.54,-41.91,;.8,-41.14,;.79,-39.59,;-.54,-38.83,;-3.2,-34.19,;-3.19,-32.65,;-4.53,-34.96,;-5.86,-34.19,;-7.2,-34.96,;-8.53,-34.18,;-8.53,-32.64,;-7.19,-31.88,;-5.86,-32.65,;-7.4,-32.68,;-7,-34.18,;6.11,-36.53,;6.11,-34.99,;7.44,-37.3,)| Show InChI InChI=1S/C30H30ClF3N4O4/c31-23-15-21(30(32,33)34)8-9-26(23)42-29-22(7-4-12-35-29)27(39)37-25(18-41-17-19-5-2-1-3-6-19)28(40)36-24-16-38-13-10-20(24)11-14-38/h1-9,12,15,20,24-25H,10-11,13-14,16-18H2,(H,36,40)(H,37,39)/t24-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50028256

(CHEMBL3354636)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(cc1F)-c1ccccc1 |r| Show InChI InChI=1S/C24H32FNO5/c25-20-13-18(17-7-3-1-4-8-17)9-10-19(20)16-31-12-6-2-5-11-26-14-22(28)24(30)23(29)21(26)15-27/h1,3-4,7-10,13,21-24,27-30H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM339784
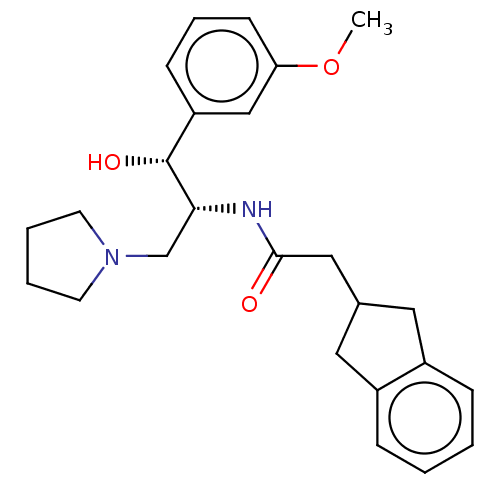
(US10202340, Example 3)Show SMILES COc1cccc(c1)[C@@H](O)[C@@H](CN1CCCC1)NC(=O)CC1Cc2ccccc2C1 |r| Show InChI InChI=1S/C25H32N2O3/c1-30-22-10-6-9-21(16-22)25(29)23(17-27-11-4-5-12-27)26-24(28)15-18-13-19-7-2-3-8-20(19)14-18/h2-3,6-10,16,18,23,25,29H,4-5,11-15,17H2,1H3,(H,26,28)/t23-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
| Assay Description
Inhibition of GlcCer production in whole wild-type MDCKII cells. |
US Patent US10202340 (2019)
BindingDB Entry DOI: 10.7270/Q21V5H30 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM367616

(N-((1R,2R)-1-(3-chloro-4- cyclopropoxyphenyl)-1-hy...)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)C1=NOCc2cc(ccc12)-c1ccc(F)cc1)c1ccc(OC2CC2)c(Cl)c1 |r,t:13| Show InChI InChI=1S/C31H31ClFN3O4/c32-26-16-21(6-12-28(26)40-24-9-10-24)30(37)27(17-36-13-1-2-14-36)34-31(38)29-25-11-5-20(15-22(25)18-39-35-29)19-3-7-23(33)8-4-19/h3-8,11-12,15-16,24,27,30,37H,1-2,9-10,13-14,17-18H2,(H,34,38)/t27-,30-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
This assay was modified based on the study by Larsen et al. (J. Lipid Res. 2011, 53, 282). Madin-Darby canine kidney (MDCK) cell lysate was prepared ... |
J Med Chem 51: 1319-23 (2008)
BindingDB Entry DOI: 10.7270/Q2G44SK0 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM367617
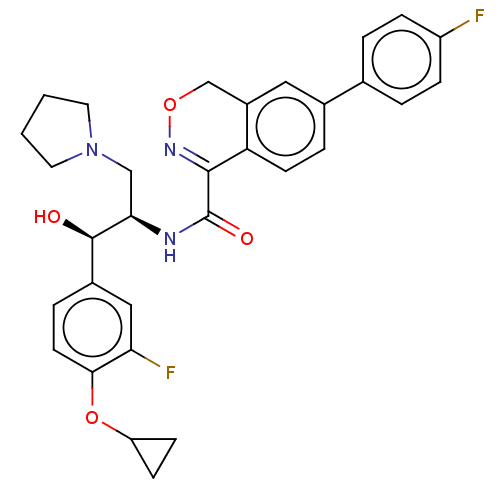
(N-((1R,2R)-1-(4-cyclopropoxy-3- fluorophenyl)-1-hy...)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)C1=NOCc2cc(ccc12)-c1ccc(F)cc1)c1ccc(OC2CC2)c(F)c1 |r,t:13| Show InChI InChI=1S/C31H31F2N3O4/c32-23-7-3-19(4-8-23)20-5-11-25-22(15-20)18-39-35-29(25)31(38)34-27(17-36-13-1-2-14-36)30(37)21-6-12-28(26(33)16-21)40-24-9-10-24/h3-8,11-12,15-16,24,27,30,37H,1-2,9-10,13-14,17-18H2,(H,34,38)/t27-,30-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
This assay was modified based on the study by Larsen et al. (J. Lipid Res. 2011, 53, 282). Madin-Darby canine kidney (MDCK) cell lysate was prepared ... |
J Med Chem 51: 1319-23 (2008)
BindingDB Entry DOI: 10.7270/Q2G44SK0 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM367618

(N-((1R,2R)-1-(8-fluoro-2,3- dihydrobenzo[b][1,4]di...)Show SMILES O[C@@H]([C@@H](CN1CCCC1)NC(=O)C1=NOCc2cc(ccc12)-c1ccc(F)cc1)c1cc(F)c2OCCOc2c1 |r,t:13| Show InChI InChI=1S/C30H29F2N3O5/c31-22-6-3-18(4-7-22)19-5-8-23-21(13-19)17-40-34-27(23)30(37)33-25(16-35-9-1-2-10-35)28(36)20-14-24(32)29-26(15-20)38-11-12-39-29/h3-8,13-15,25,28,36H,1-2,9-12,16-17H2,(H,33,37)/t25-,28-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
This assay was modified based on the study by Larsen et al. (J. Lipid Res. 2011, 53, 282). Madin-Darby canine kidney (MDCK) cell lysate was prepared ... |
J Med Chem 51: 1319-23 (2008)
BindingDB Entry DOI: 10.7270/Q2G44SK0 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM367622

(N-((1R,2R)-3-(azetidin-1-yl)-1-(4- cyclopropoxy-3-...)Show SMILES O[C@@H]([C@@H](CN1CCC1)NC(=O)C1=NOCc2cc(ccc12)-c1ccc(F)cc1)c1ccc(OC2CC2)c(F)c1 |r,t:12| Show InChI InChI=1S/C30H29F2N3O4/c31-22-6-2-18(3-7-22)19-4-10-24-21(14-19)17-38-34-28(24)30(37)33-26(16-35-12-1-13-35)29(36)20-5-11-27(25(32)15-20)39-23-8-9-23/h2-7,10-11,14-15,23,26,29,36H,1,8-9,12-13,16-17H2,(H,33,37)/t26-,29-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
This assay was modified based on the study by Larsen et al. (J. Lipid Res. 2011, 53, 282). Madin-Darby canine kidney (MDCK) cell lysate was prepared ... |
J Med Chem 51: 1319-23 (2008)
BindingDB Entry DOI: 10.7270/Q2G44SK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data