 Found 31 hits of ic50 data for polymerid = 50000305
Found 31 hits of ic50 data for polymerid = 50000305 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201438
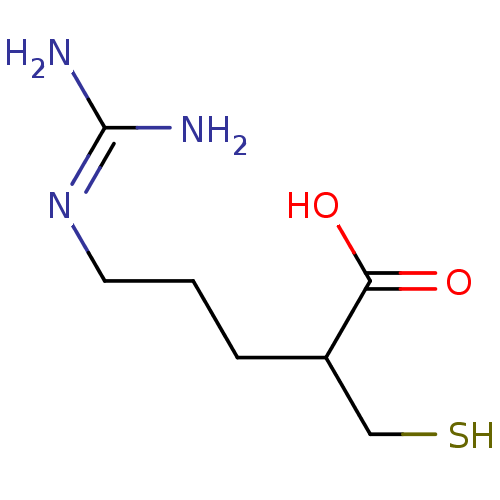
((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C7H15N3O2S/c8-7(9)10-3-1-2-5(4-13)6(11)12/h5,13H,1-4H2,(H,11,12)(H4,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201439
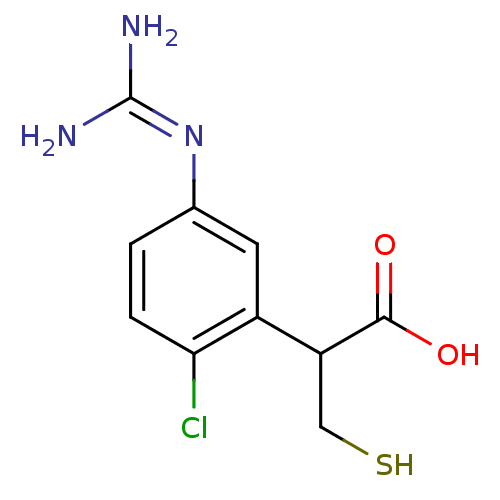
(2-(2-chloro-5-guanidinophenyl)-3-mercaptopropanoic...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(Cl)c(c1)-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C10H12ClN3O2S/c11-8-2-1-5(14-10(12)13)3-6(8)7(4-17)9(15)16/h1-3,7,17H,4H2,(H,15,16)(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201427
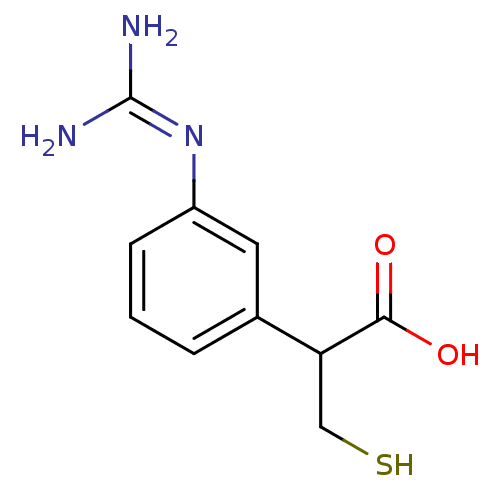
(2-(3-guanidinophenyl)-3-mercaptopropanoic acid | C...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1cccc(c1)-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C10H13N3O2S/c11-10(12)13-7-3-1-2-6(4-7)8(5-16)9(14)15/h1-4,8,16H,5H2,(H,14,15)(H4,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201427

(2-(3-guanidinophenyl)-3-mercaptopropanoic acid | C...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1cccc(c1)-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C10H13N3O2S/c11-10(12)13-7-3-1-2-6(4-7)8(5-16)9(14)15/h1-4,8,16H,5H2,(H,14,15)(H4,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201429
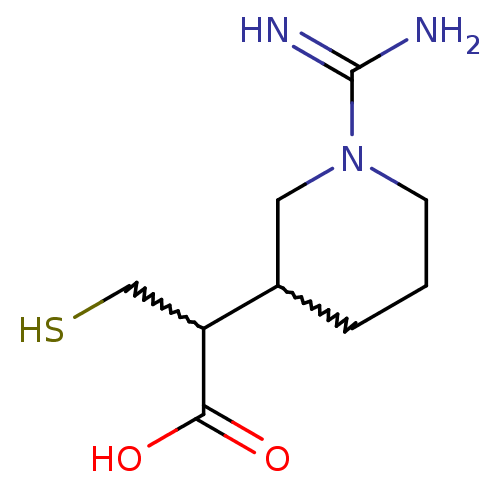
(2-(1-carbamimidoylpiperidin-3-yl)-3-mercaptopropan...)Show InChI InChI=1S/C9H17N3O2S/c10-9(11)12-3-1-2-6(4-12)7(5-15)8(13)14/h6-7,15H,1-5H2,(H3,10,11)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201429

(2-(1-carbamimidoylpiperidin-3-yl)-3-mercaptopropan...)Show InChI InChI=1S/C9H17N3O2S/c10-9(11)12-3-1-2-6(4-12)7(5-15)8(13)14/h6-7,15H,1-5H2,(H3,10,11)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201428
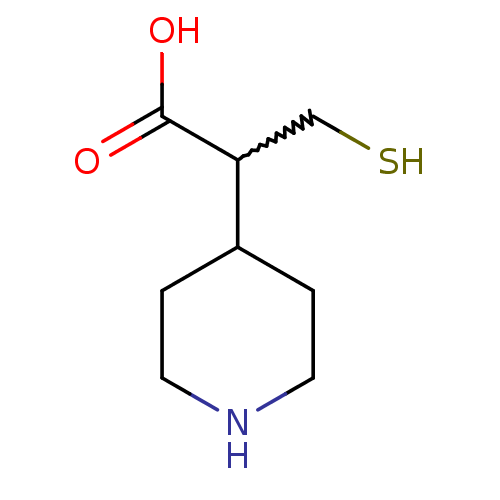
(3-mercapto-2-(piperidin-4-yl)propanoic acid | CHEM...)Show InChI InChI=1S/C8H15NO2S/c10-8(11)7(5-12)6-1-3-9-4-2-6/h6-7,9,12H,1-5H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201435
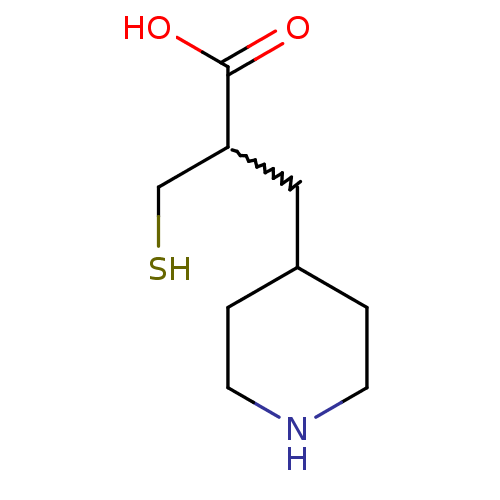
(2-(mercaptomethyl)-3-(piperidin-4-yl)propanoic aci...)Show InChI InChI=1S/C9H17NO2S/c11-9(12)8(6-13)5-7-1-3-10-4-2-7/h7-8,10,13H,1-6H2,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201431
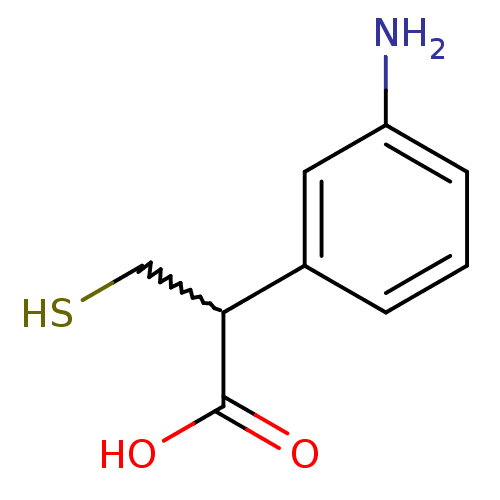
(2-(3-aminophenyl)-3-mercaptopropanoic acid | CHEMB...)Show InChI InChI=1S/C9H11NO2S/c10-7-3-1-2-6(4-7)8(5-13)9(11)12/h1-4,8,13H,5,10H2,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201437
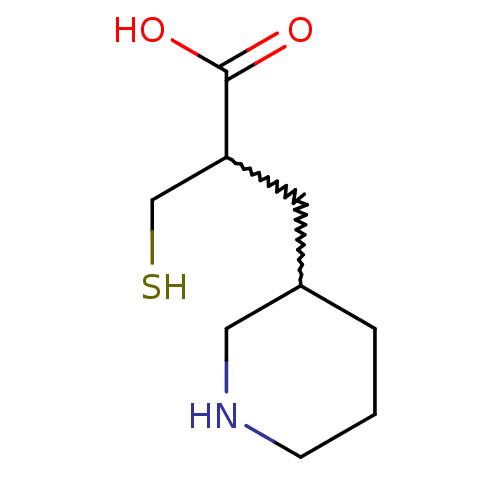
(2-(mercaptomethyl)-3-(piperidin-3-yl)propanoic aci...)Show InChI InChI=1S/C9H17NO2S/c11-9(12)8(6-13)4-7-2-1-3-10-5-7/h7-8,10,13H,1-6H2,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201437

(2-(mercaptomethyl)-3-(piperidin-3-yl)propanoic aci...)Show InChI InChI=1S/C9H17NO2S/c11-9(12)8(6-13)4-7-2-1-3-10-5-7/h7-8,10,13H,1-6H2,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201430
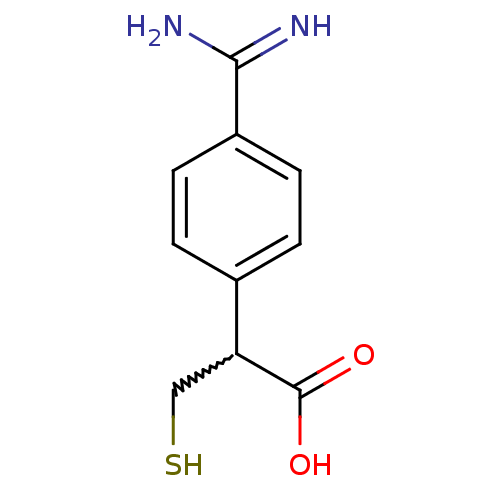
(2-(4-carbamimidoylphenyl)-3-mercaptopropanoic acid...)Show InChI InChI=1S/C10H12N2O2S/c11-9(12)7-3-1-6(2-4-7)8(5-15)10(13)14/h1-4,8,15H,5H2,(H3,11,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201434
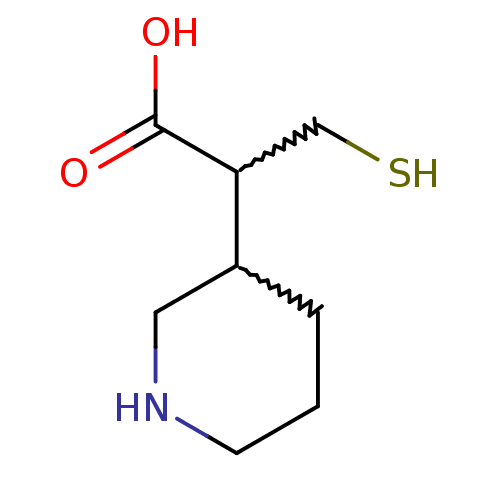
(3-mercapto-2-(piperidin-3-yl)propanoic acid | CHEM...)Show InChI InChI=1S/C8H15NO2S/c10-8(11)7(5-12)6-2-1-3-9-4-6/h6-7,9,12H,1-5H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201434

(3-mercapto-2-(piperidin-3-yl)propanoic acid | CHEM...)Show InChI InChI=1S/C8H15NO2S/c10-8(11)7(5-12)6-2-1-3-9-4-6/h6-7,9,12H,1-5H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50089687
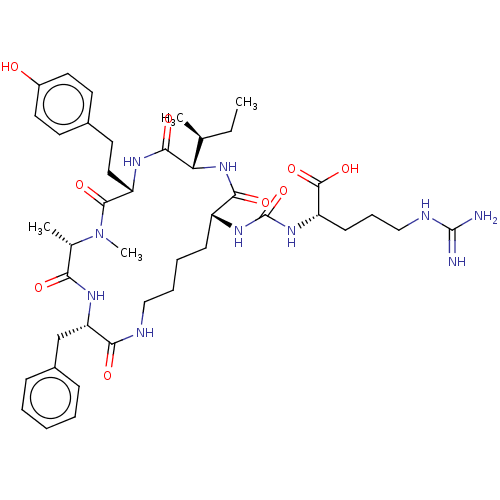
(Anabaenopeptin F)Show SMILES [H][C@]1(NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C42H62N10O9/c1-5-25(2)34-38(57)47-31(21-18-27-16-19-29(53)20-17-27)39(58)52(4)26(3)35(54)48-33(24-28-12-7-6-8-13-28)36(55)45-22-10-9-14-30(37(56)51-34)49-42(61)50-32(40(59)60)15-11-23-46-41(43)44/h6-8,12-13,16-17,19-20,25-26,30-34,53H,5,9-11,14-15,18,21-24H2,1-4H3,(H,45,55)(H,47,57)(H,48,54)(H,51,56)(H,59,60)(H4,43,44,46)(H2,49,50,61)/t25-,26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPN (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201433
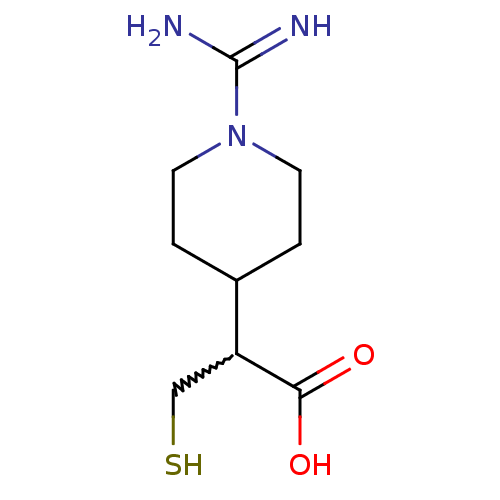
(2-(1-carbamimidoylpiperidin-4-yl)-3-mercaptopropan...)Show InChI InChI=1S/C9H17N3O2S/c10-9(11)12-3-1-6(2-4-12)7(5-15)8(13)14/h6-7,15H,1-5H2,(H3,10,11)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201436
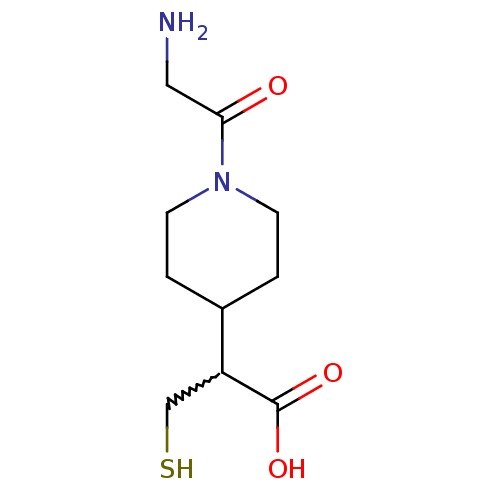
(2-(1-(2-aminoacetyl)piperidin-4-yl)-3-mercaptoprop...)Show InChI InChI=1S/C10H18N2O3S/c11-5-9(13)12-3-1-7(2-4-12)8(6-16)10(14)15/h7-8,16H,1-6,11H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50089688
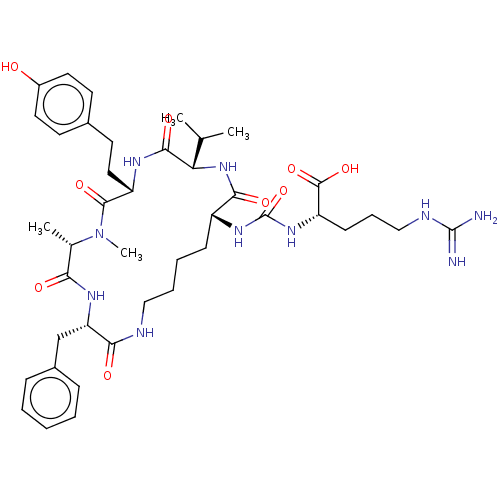
(ANABAENOPEPTIN B)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C41H60N10O9/c1-24(2)33-37(56)46-30(20-17-26-15-18-28(52)19-16-26)38(57)51(4)25(3)34(53)47-32(23-27-11-6-5-7-12-27)35(54)44-21-9-8-13-29(36(55)50-33)48-41(60)49-31(39(58)59)14-10-22-45-40(42)43/h5-7,11-12,15-16,18-19,24-25,29-33,52H,8-10,13-14,17,20-23H2,1-4H3,(H,44,54)(H,46,56)(H,47,53)(H,50,55)(H,58,59)(H4,42,43,45)(H2,48,49,60)/t25-,29+,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPN (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50089686
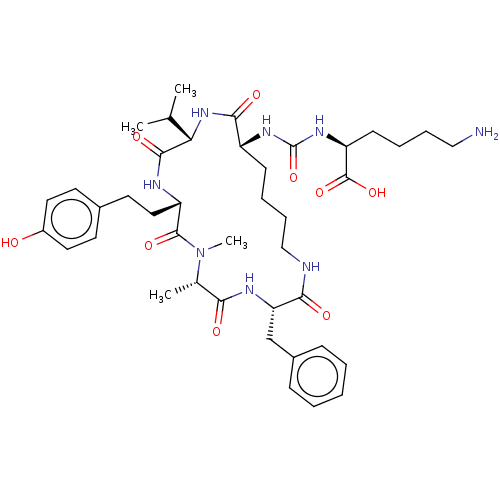
(CHEMBL3577334)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](CCc2ccc(O)cc2)NC1=O)NC(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C41H60N8O9/c1-25(2)34-38(54)44-31(21-18-27-16-19-29(50)20-17-27)39(55)49(4)26(3)35(51)45-33(24-28-12-6-5-7-13-28)36(52)43-23-11-9-14-30(37(53)48-34)46-41(58)47-32(40(56)57)15-8-10-22-42/h5-7,12-13,16-17,19-20,25-26,30-34,50H,8-11,14-15,18,21-24,42H2,1-4H3,(H,43,52)(H,44,54)(H,45,51)(H,48,53)(H,56,57)(H2,46,47,58)/t26-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research
Curated by ChEMBL
| Assay Description
Inhibition of CPN (unknown origin) |
J Med Chem 58: 4839-44 (2015)
Article DOI: 10.1021/jm501840b
BindingDB Entry DOI: 10.7270/Q24B3314 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201432
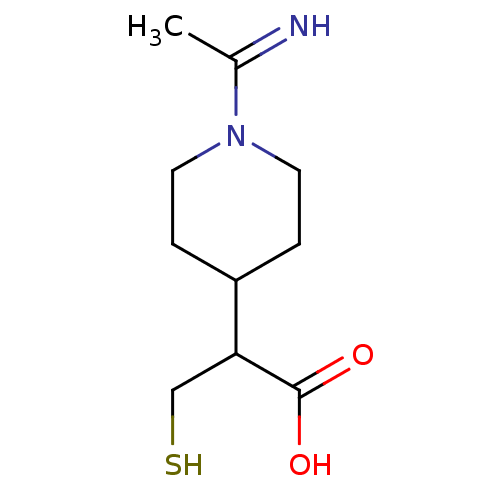
(2-(1-(1-iminoethyl)piperidin-4-yl)-3-mercaptopropa...)Show InChI InChI=1S/C10H18N2O2S/c1-7(11)12-4-2-8(3-5-12)9(6-15)10(13)14/h8-9,11,15H,2-6H2,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N |
Bioorg Med Chem Lett 17: 1349-54 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.078
BindingDB Entry DOI: 10.7270/Q2RJ4J5B |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50275212
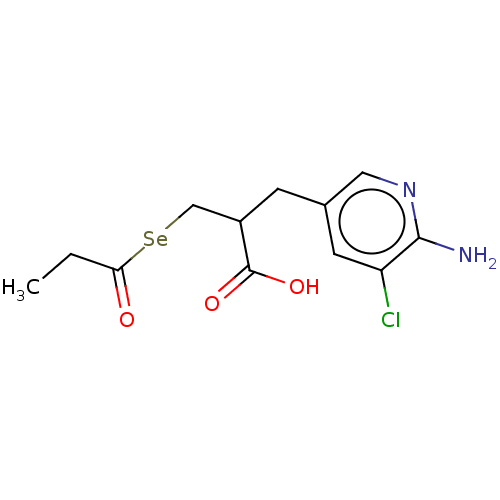
(CHEMBL4127473)Show SMILES [#6]-[#6]-[#6](=O)[Se;v2][#6]-[#6](-[#6]-c1cnc(-[#7])c(Cl)c1)-[#6](-[#8])=O Show InChI InChI=1S/C12H15ClN2O3Se/c1-2-10(16)19-6-8(12(17)18)3-7-4-9(13)11(14)15-5-7/h4-5,8H,2-3,6H2,1H3,(H2,14,15)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N preincubated for 10 mins followed by hippuryl-lysine substrate addition measured after 30 mins in presence of ... |
Bioorg Med Chem Lett 28: 2256-2260 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.042
BindingDB Entry DOI: 10.7270/Q25M686N |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50275212

(CHEMBL4127473)Show SMILES [#6]-[#6]-[#6](=O)[Se;v2][#6]-[#6](-[#6]-c1cnc(-[#7])c(Cl)c1)-[#6](-[#8])=O Show InChI InChI=1S/C12H15ClN2O3Se/c1-2-10(16)19-6-8(12(17)18)3-7-4-9(13)11(14)15-5-7/h4-5,8H,2-3,6H2,1H3,(H2,14,15)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase N preincubated for 10 mins followed by hippuryl-lysine substrate addition measured after 30 mins in absence of D... |
Bioorg Med Chem Lett 28: 2256-2260 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.042
BindingDB Entry DOI: 10.7270/Q25M686N |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008277
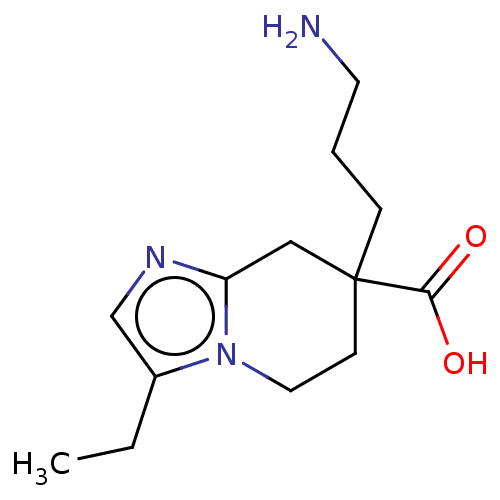
(CHEMBL3235138)Show InChI InChI=1S/C13H21N3O2/c1-2-10-9-15-11-8-13(12(17)18,4-3-6-14)5-7-16(10)11/h9H,2-8,14H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008276
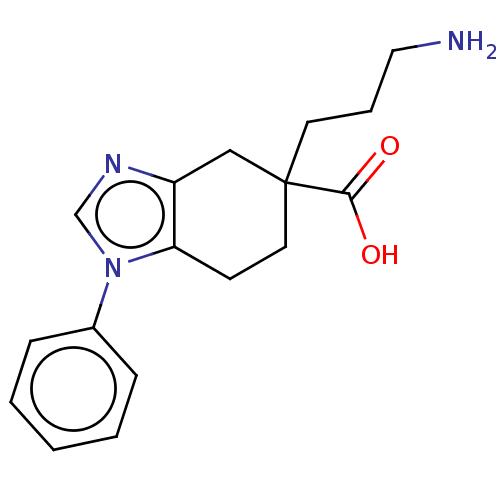
(CHEMBL3235137)Show InChI InChI=1S/C17H21N3O2/c18-10-4-8-17(16(21)22)9-7-15-14(11-17)19-12-20(15)13-5-2-1-3-6-13/h1-3,5-6,12H,4,7-11,18H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008275
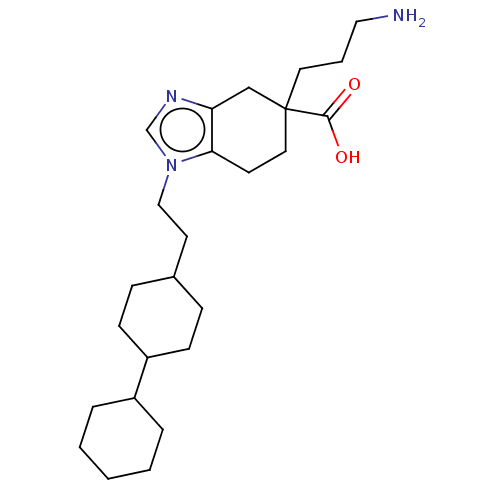
(CHEMBL3235136)Show SMILES NCCCC1(CCc2c(C1)ncn2CCC1CCC(CC1)C1CCCCC1)C(O)=O |(12.03,-18.01,;11.64,-19.5,;10.15,-19.91,;9.76,-21.4,;8.28,-21.8,;8.28,-20.26,;6.95,-19.48,;5.61,-20.25,;5.62,-21.8,;6.95,-22.56,;4.15,-22.29,;3.24,-21.04,;4.14,-19.78,;3.66,-18.32,;4.68,-17.17,;4.2,-15.71,;5.22,-14.56,;4.74,-13.1,;3.23,-12.78,;2.2,-13.93,;2.68,-15.4,;2.75,-11.32,;3.78,-10.18,;3.31,-8.72,;1.8,-8.39,;.77,-9.54,;1.25,-11.01,;9.04,-23.13,;8.26,-24.46,;10.58,-23.14,)| Show InChI InChI=1S/C25H41N3O2/c26-15-4-13-25(24(29)30)14-11-23-22(17-25)27-18-28(23)16-12-19-7-9-21(10-8-19)20-5-2-1-3-6-20/h18-21H,1-17,26H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008274
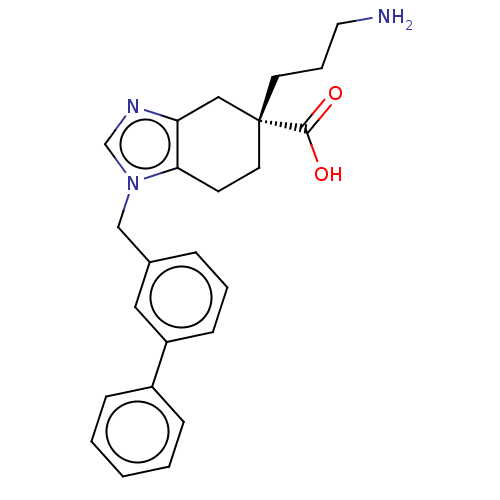
(CHEMBL3235135)Show SMILES NCCC[C@]1(CCc2c(C1)ncn2Cc1cccc(c1)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O2/c25-13-5-11-24(23(28)29)12-10-22-21(15-24)26-17-27(22)16-18-6-4-9-20(14-18)19-7-2-1-3-8-19/h1-4,6-9,14,17H,5,10-13,15-16,25H2,(H,28,29)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008279
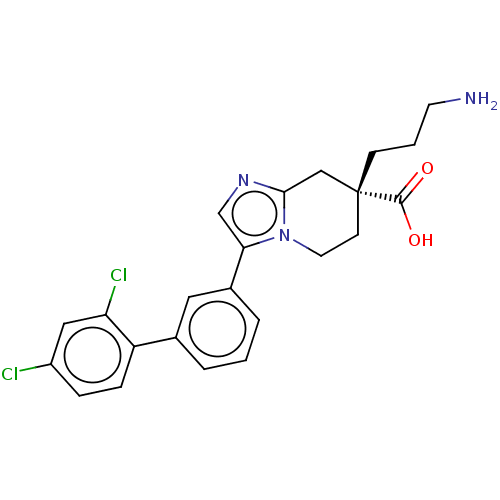
(CHEMBL3235140)Show SMILES Cl.NCCC[C@]1(CCn2c(C1)ncc2-c1cccc(c1)-c1ccc(Cl)cc1Cl)C(O)=O |r| Show InChI InChI=1S/C23H23Cl2N3O2.ClH/c24-17-5-6-18(19(25)12-17)15-3-1-4-16(11-15)20-14-27-21-13-23(22(29)30,7-2-9-26)8-10-28(20)21;/h1,3-6,11-12,14H,2,7-10,13,26H2,(H,29,30);1H/t23-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008272
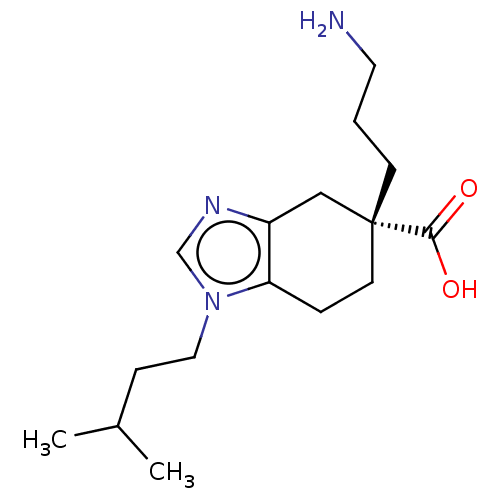
(CHEMBL3235133)Show SMILES Cl.CC(C)CCn1cnc2C[C@@](CCCN)(CCc12)C(O)=O |r| Show InChI InChI=1S/C16H27N3O2.ClH/c1-12(2)5-9-19-11-18-13-10-16(15(20)21,6-3-8-17)7-4-14(13)19;/h11-12H,3-10,17H2,1-2H3,(H,20,21);1H/t16-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008271
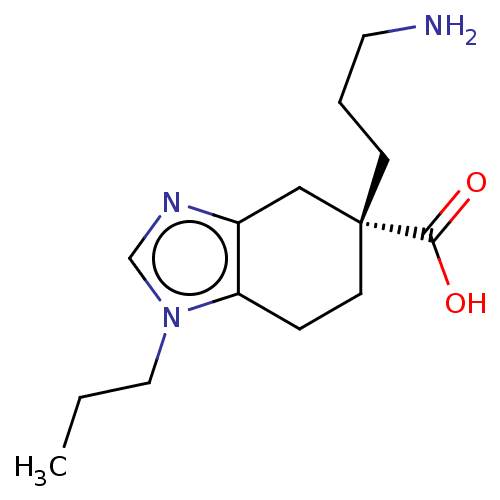
(CHEMBL3235132)Show InChI InChI=1S/C14H23N3O2/c1-2-8-17-10-16-11-9-14(13(18)19,5-3-7-15)6-4-12(11)17/h10H,2-9,15H2,1H3,(H,18,19)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008278
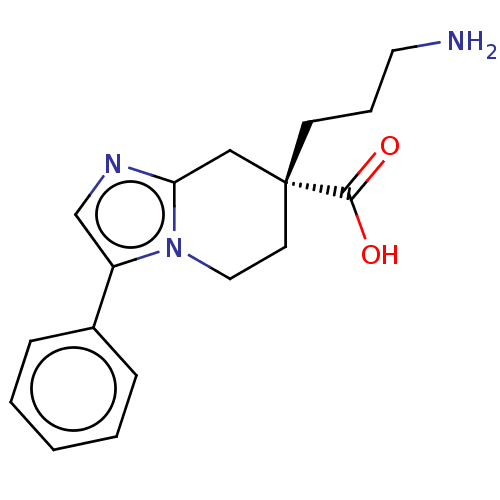
(CHEMBL3235139)Show SMILES NCCC[C@]1(CCn2c(C1)ncc2-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C17H21N3O2/c18-9-4-7-17(16(21)22)8-10-20-14(12-19-15(20)11-17)13-5-2-1-3-6-13/h1-3,5-6,12H,4,7-11,18H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50008273
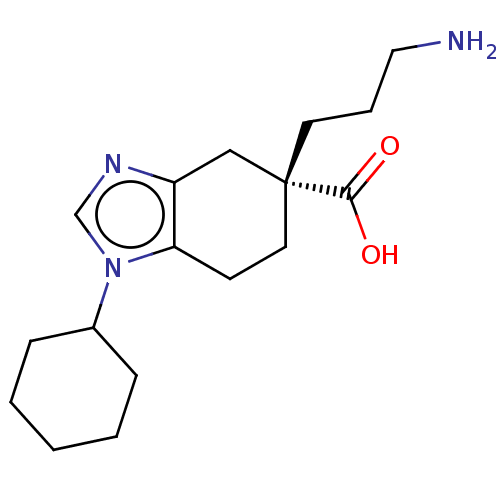
(CHEMBL3235134)Show InChI InChI=1S/C17H27N3O2/c18-10-4-8-17(16(21)22)9-7-15-14(11-17)19-12-20(15)13-5-2-1-3-6-13/h12-13H,1-11,18H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of carboxypeptidase N (unknown origin) |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data