

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695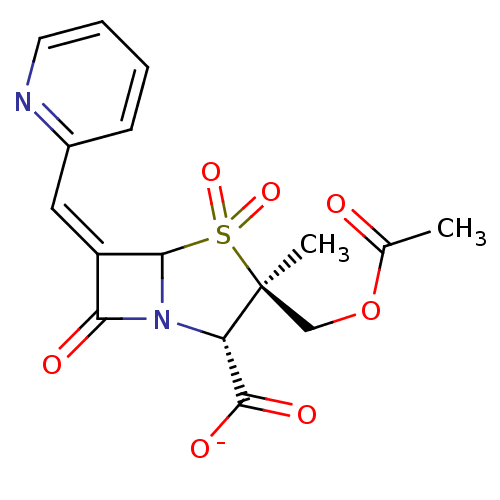 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079686 (CHEMBL292093 | disodium (2S,3R,5R,6Z)-3-methyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079689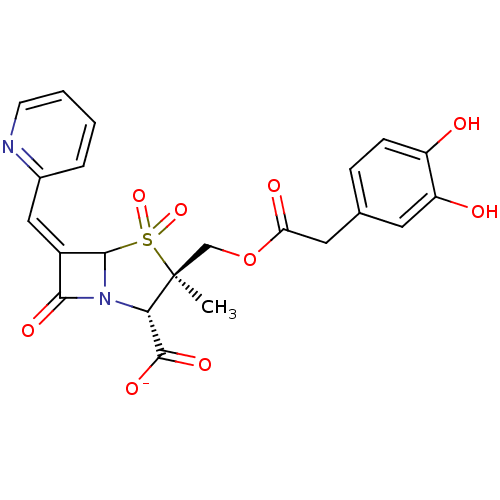 (CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50033680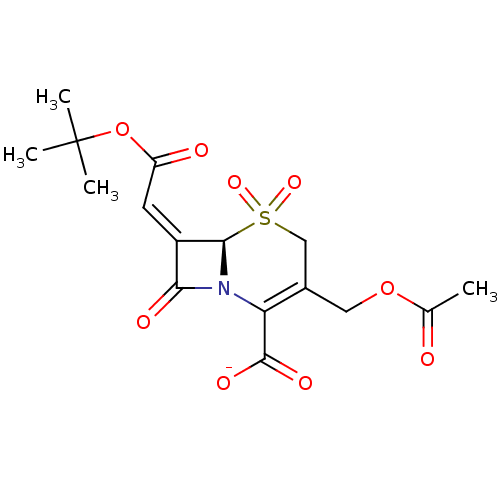 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212641 (Brobactam) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 46.4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079689 (CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212643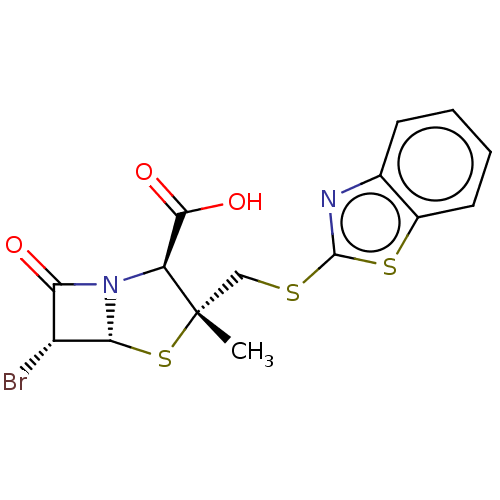 (CHEMBL308516) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 71.8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Penicillinase from Staphylococcus aureus TH-14 using piperacillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088831 (CHEMBL353613 | Sodium; 3-((E)-2-carbamoyl-vinyl)-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of class A beta-lactamase derived from Staphylococcus aureus strain PC1 | Bioorg Med Chem Lett 10: 853-7 (2000) BindingDB Entry DOI: 10.7270/Q2HQ3Z41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212634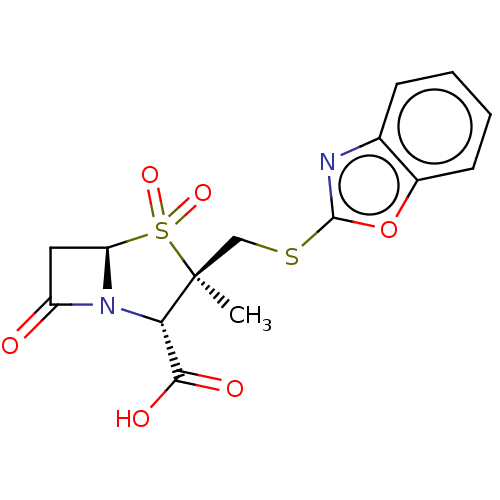 (CHEMBL309009) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079684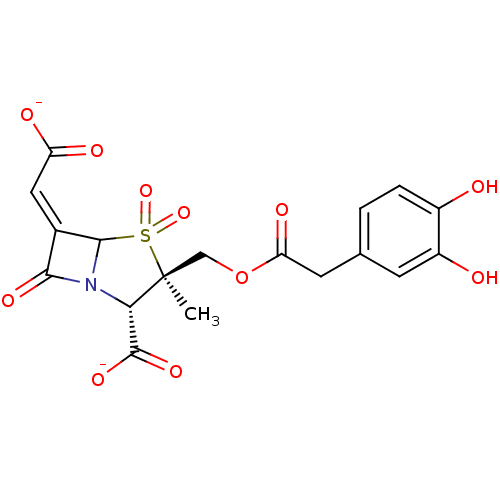 (CHEMBL305361 | disodium (2S,3R,5R,6Z)-3-({[(3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212642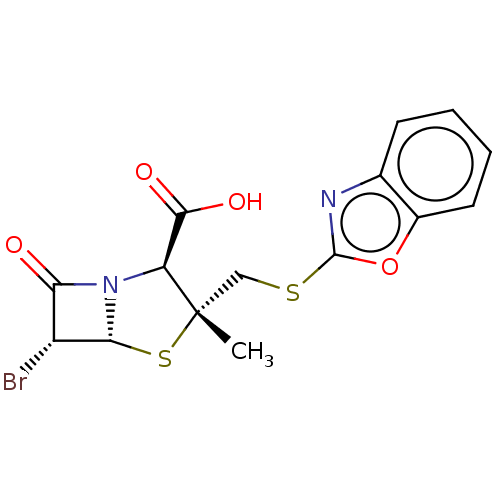 (CHEMBL70269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212636 (CHEMBL310221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Russell beta-lactamase assessed as inhibition of nitrocefin hydrolysis pre-incubated for 5 mins by microtiter pla... | J Nat Prod 57: 654-7 (1994) Article DOI: 10.1021/np50107a016 BindingDB Entry DOI: 10.7270/Q2SQ935W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079685 (CHEMBL61762 | disodium (2S,3R,5R,6Z)-3-[(acetyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212638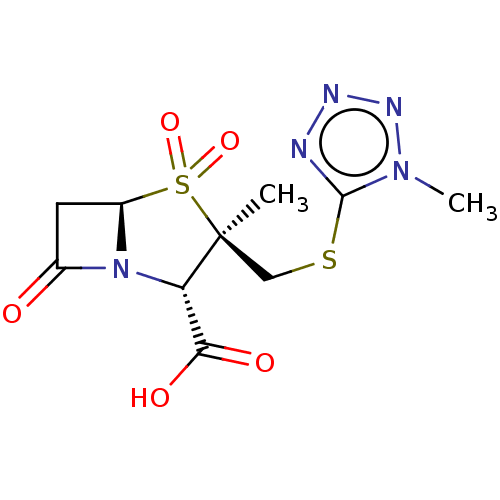 (CHEMBL302512) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079682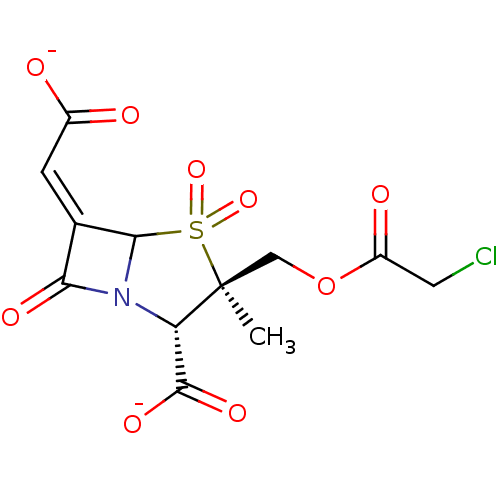 (CHEMBL64793 | disodium (2S,3R,5R,6Z)-3-{[(chloroac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088825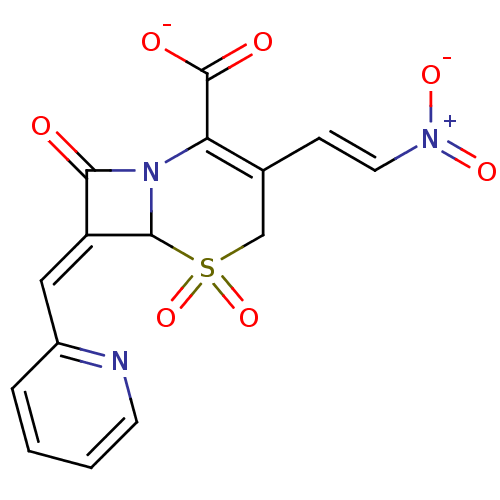 (CHEMBL353422 | Sodium; 3-((E)-2-nitro-vinyl)-5,5,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of class A beta-lactamase derived from Staphylococcus aureus strain PC1 | Bioorg Med Chem Lett 10: 853-7 (2000) BindingDB Entry DOI: 10.7270/Q2HQ3Z41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079692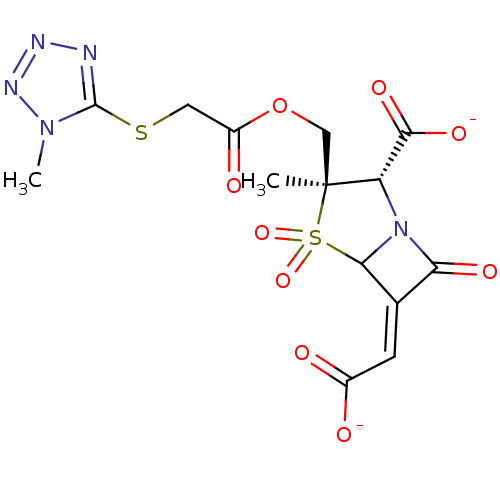 (CHEMBL292506 | Disodium; (2S,3R)-6-[1-carboxy-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Staphylococcus aureus PCI | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50021956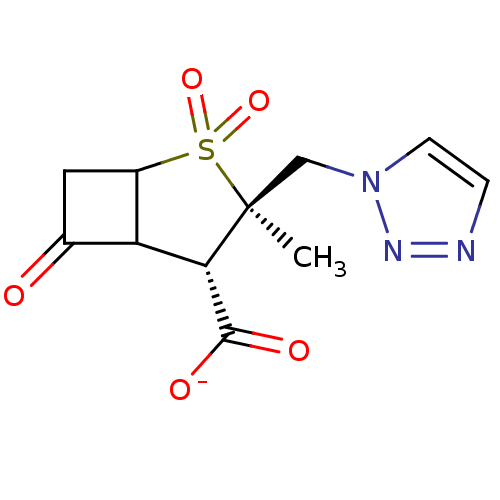 (CHEMBL285387 | Sodium; 3-methyl-2,2,6-trioxo-3-[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Penicillinase from Staphylococcus aureus TH-14 using piperacillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212639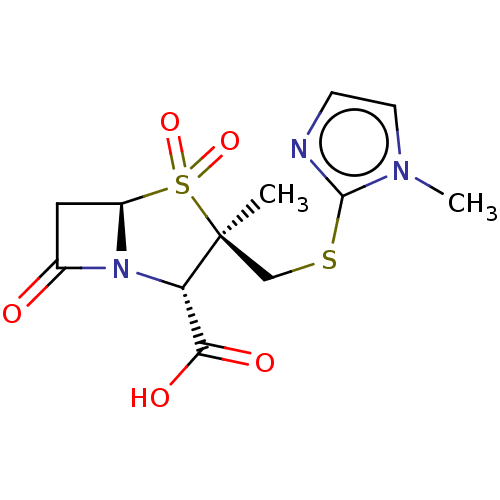 (CHEMBL302241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50157692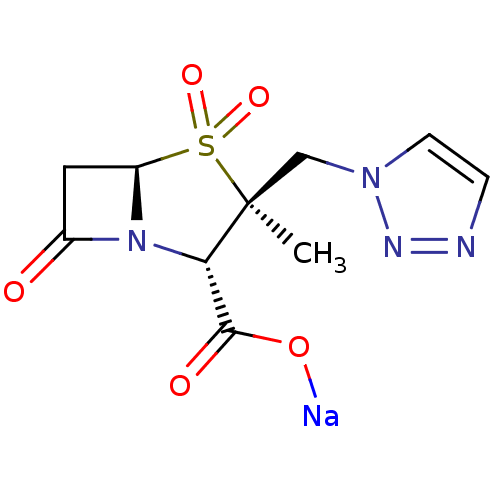 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50421413 (CL-307579 | TAZOBACTAM SODIUM | Tazocillin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088834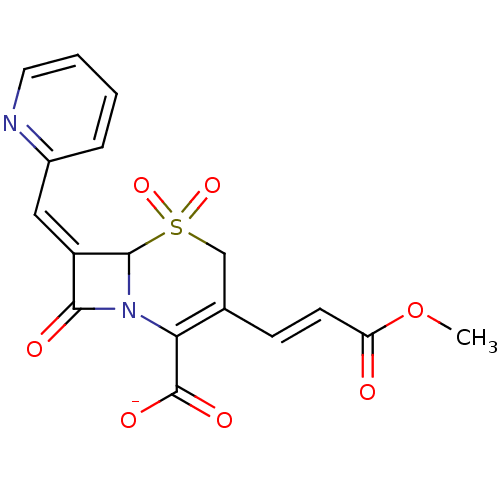 (CHEMBL169392 | Sodium; 3-((E)-2-methoxycarbonyl-vi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of class A beta-lactamase derived from Staphylococcus aureus strain PC1 | Bioorg Med Chem Lett 10: 853-7 (2000) BindingDB Entry DOI: 10.7270/Q2HQ3Z41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079690 (CHEMBL304709 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212645 (CHEMBL305908) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079684 (CHEMBL305361 | disodium (2S,3R,5R,6Z)-3-({[(3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212646 (CHEMBL72972) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 419 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212640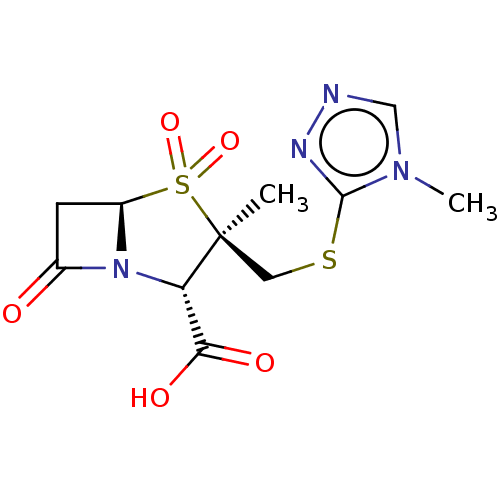 (CHEMBL423309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 461 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Staphylococcus aureus PCI | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212644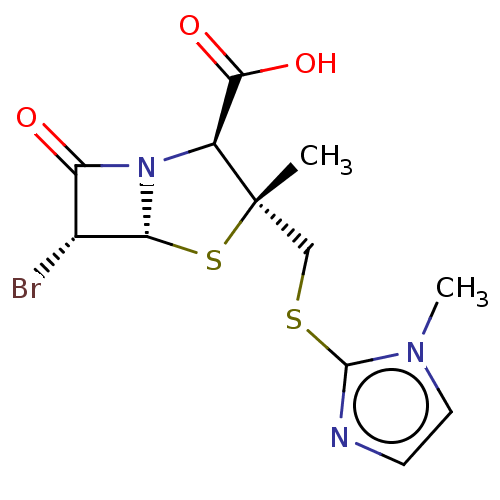 (CHEMBL73450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 509 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079686 (CHEMBL292093 | disodium (2S,3R,5R,6Z)-3-methyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212637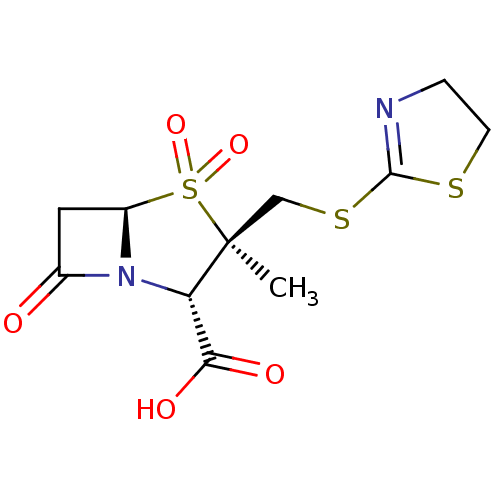 (CHEMBL72046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079680 (CHEMBL61760 | disodium (2S,3R,5R,6Z)-3-[(formyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079692 (CHEMBL292506 | Disodium; (2S,3R)-6-[1-carboxy-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079689 (CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079685 (CHEMBL61762 | disodium (2S,3R,5R,6Z)-3-[(acetyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088827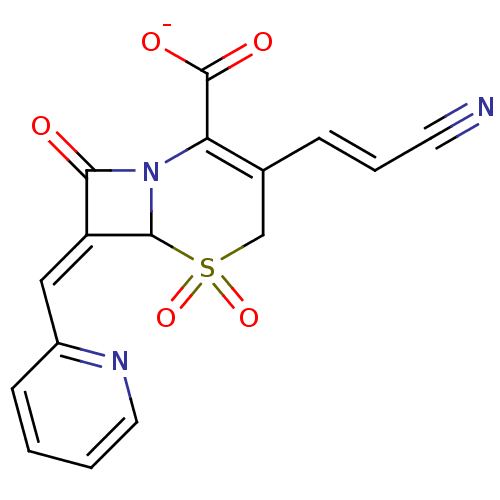 (CHEMBL355558 | Sodium; 3-((E)-2-cyano-vinyl)-5,5,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of class A beta-lactamase derived from Staphylococcus aureus strain PC1 | Bioorg Med Chem Lett 10: 853-7 (2000) BindingDB Entry DOI: 10.7270/Q2HQ3Z41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088814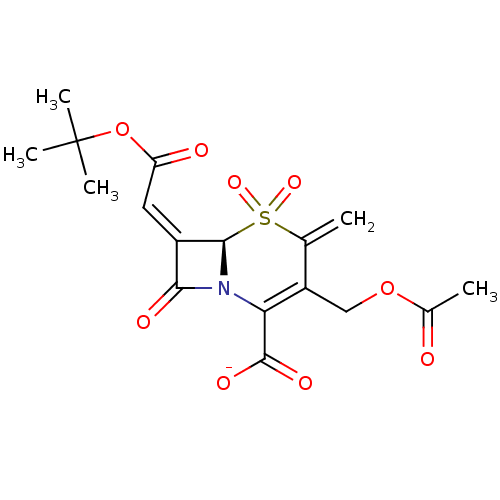 (CHEMBL169515 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 932 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088806 (CHEMBL169016 | Sodium; (R)-7-[1-tert-butoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088816 (CHEMBL167539 | Sodium; 3-((Z)-2-chloro-2-methoxyca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of class A beta-lactamase derived from Staphylococcus aureus strain PC1 | Bioorg Med Chem Lett 10: 853-7 (2000) BindingDB Entry DOI: 10.7270/Q2HQ3Z41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50212635 (CHEMBL415266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |