 Found 236 hits of ic50 data for polymerid = 50000561,50002182,50002390
Found 236 hits of ic50 data for polymerid = 50000561,50002182,50002390 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018507
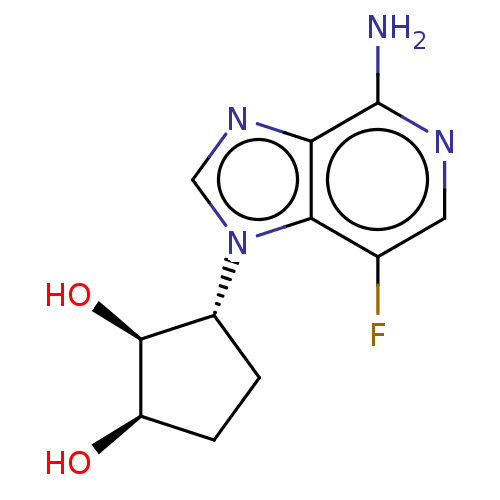
(CHEMBL3290657)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006218
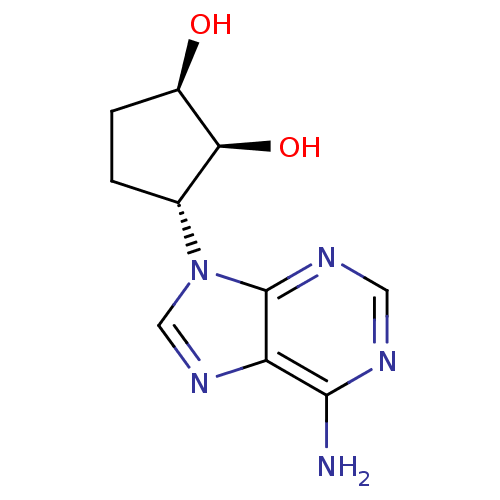
((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...)Show InChI InChI=1S/C10H13N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h3-6,8,16-17H,1-2H2,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018501
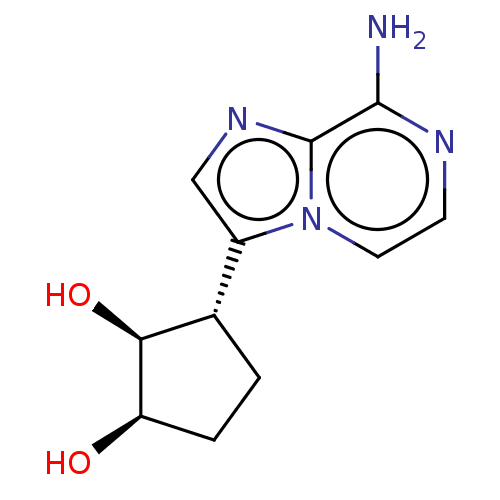
(CHEMBL3290650)Show SMILES Nc1nccn2c(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H14N4O2/c12-10-11-14-5-7(15(11)4-3-13-10)6-1-2-8(16)9(6)17/h3-6,8-9,16-17H,1-2H2,(H2,12,13)/t6-,8+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018508
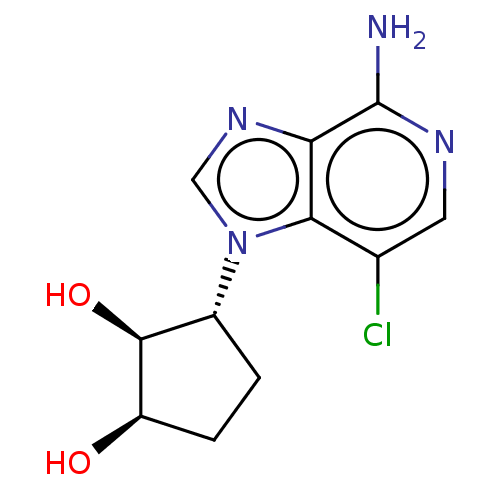
(CHEMBL3290658)Show SMILES Nc1ncc(Cl)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13ClN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018533
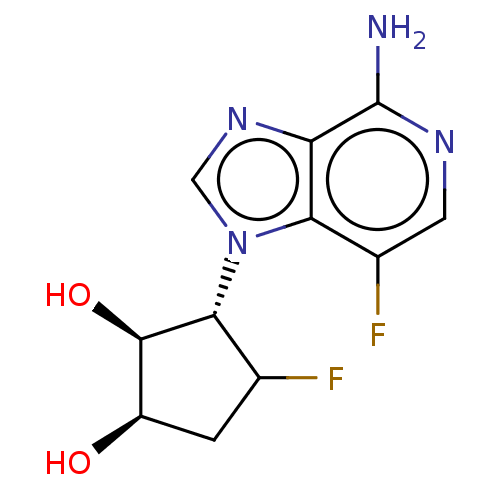
(CHEMBL3290668)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C(F)C[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(18)10(19)9(4)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4?,6-,9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050862
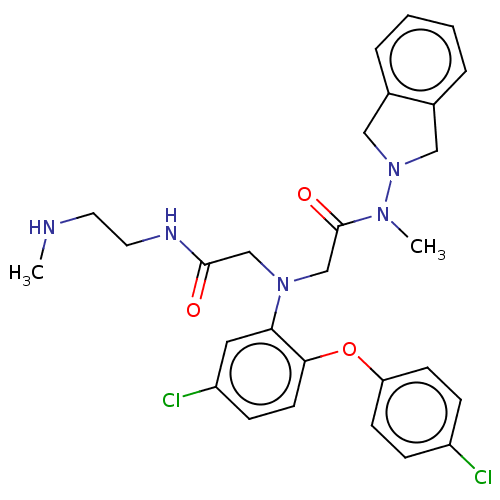
(CHEMBL3322562)Show SMILES Cl.CNCCNC(=O)CN(CC(=O)N(C)N1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H31Cl2N5O3.ClH/c1-31-13-14-32-27(36)18-34(19-28(37)33(2)35-16-20-5-3-4-6-21(20)17-35)25-15-23(30)9-12-26(25)38-24-10-7-22(29)8-11-24;/h3-12,15,31H,13-14,16-19H2,1-2H3,(H,32,36);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050862

(CHEMBL3322562)Show SMILES Cl.CNCCNC(=O)CN(CC(=O)N(C)N1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H31Cl2N5O3.ClH/c1-31-13-14-32-27(36)18-34(19-28(37)33(2)35-16-20-5-3-4-6-21(20)17-35)25-15-23(30)9-12-26(25)38-24-10-7-22(29)8-11-24;/h3-12,15,31H,13-14,16-19H2,1-2H3,(H,32,36);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 3: 663-666 (1993)
Article DOI: 10.1016/S0960-894X(01)81249-X
BindingDB Entry DOI: 10.7270/Q2X92BS9 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50405728
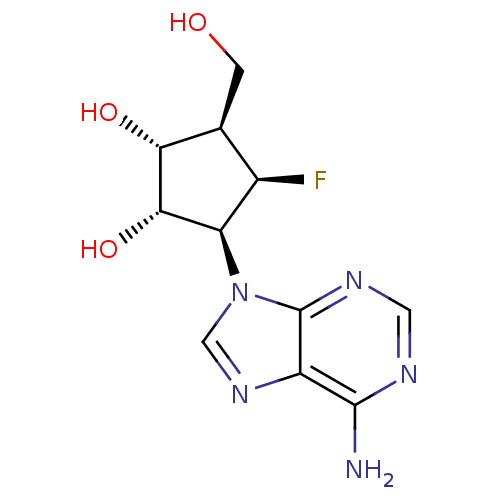
(CHEMBL2115462)Show SMILES Nc1ncnc2n(cnc12)[C@H]1[C@H](O)[C@H](O)[C@@H](CO)[C@H]1F |r| Show InChI InChI=1S/C11H14FN5O3/c12-5-4(1-18)8(19)9(20)7(5)17-3-16-6-10(13)14-2-15-11(6)17/h2-5,7-9,18-20H,1H2,(H2,13,14,15)/t4-,5+,7+,8+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-homocysteine (AdoHcy)hydrolase from rabbit erythrocytes |
J Med Chem 31: 1798-804 (1988)
BindingDB Entry DOI: 10.7270/Q2VX0H4P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50105901
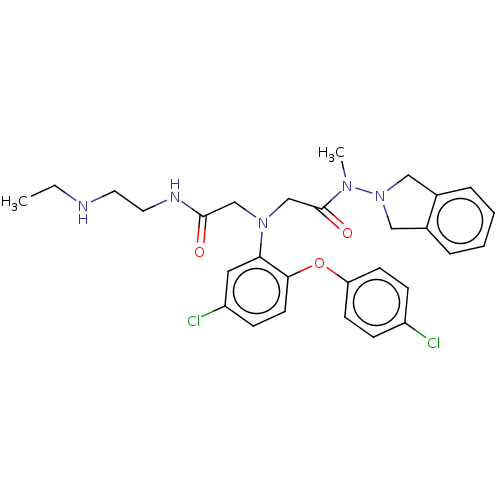
(CHEMBL3597832)Show SMILES Cl.CCNCCNC(=O)CN(CC(=O)N(C)N1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H33Cl2N5O3.ClH/c1-3-32-14-15-33-28(37)19-35(20-29(38)34(2)36-17-21-6-4-5-7-22(21)18-36)26-16-24(31)10-13-27(26)39-25-11-8-23(30)9-12-25;/h4-13,16,32H,3,14-15,17-20H2,1-2H3,(H,33,37);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018514
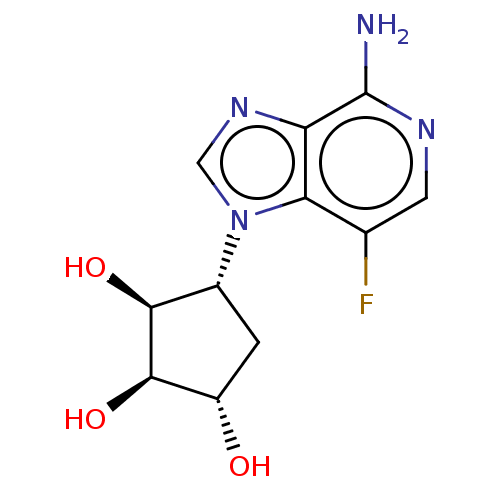
(CHEMBL3290663)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O3/c12-4-2-14-11(13)7-8(4)16(3-15-7)5-1-6(17)10(19)9(5)18/h2-3,5-6,9-10,17-19H,1H2,(H2,13,14)/t5-,6+,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050877
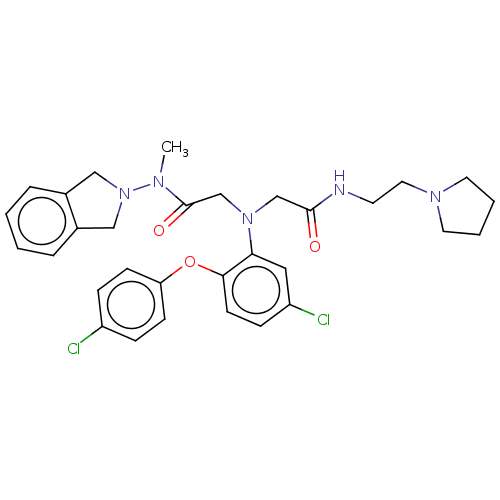
(CHEMBL3322547)Show SMILES CN(N1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H35Cl2N5O3/c1-35(38-19-23-6-2-3-7-24(23)20-38)31(40)22-37(21-30(39)34-14-17-36-15-4-5-16-36)28-18-26(33)10-13-29(28)41-27-11-8-25(32)9-12-27/h2-3,6-13,18H,4-5,14-17,19-22H2,1H3,(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050877

(CHEMBL3322547)Show SMILES CN(N1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H35Cl2N5O3/c1-35(38-19-23-6-2-3-7-24(23)20-38)31(40)22-37(21-30(39)34-14-17-36-15-4-5-16-36)28-18-26(33)10-13-29(28)41-27-11-8-25(32)9-12-27/h2-3,6-13,18H,4-5,14-17,19-22H2,1H3,(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. |
J Med Chem 35: 324-31 (1992)
BindingDB Entry DOI: 10.7270/Q2RX9B1R |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory activity against S-adenosyl-L-homocysteine hydrolase from rabbit erythrocytes |
J Med Chem 39: 2392-9 (1996)
Article DOI: 10.1021/jm950853f
BindingDB Entry DOI: 10.7270/Q2GM8B1S |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018508

(CHEMBL3290658)Show SMILES Nc1ncc(Cl)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13ClN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50281613
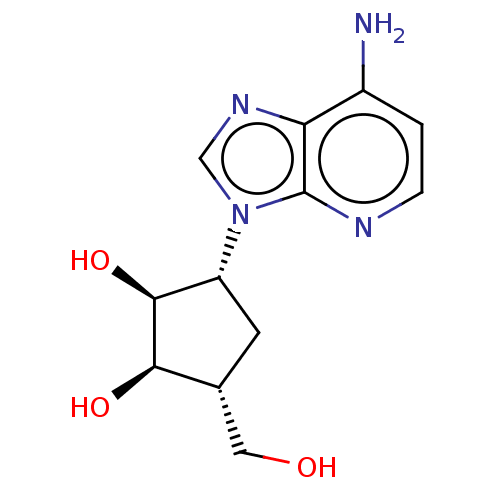
(3-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-5-hydroxyme...)Show SMILES Nc1ccnc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C12H16N4O3/c13-7-1-2-14-12-9(7)15-5-16(12)8-3-6(4-17)10(18)11(8)19/h1-2,5-6,8,10-11,17-19H,3-4H2,(H2,13,14)/t6-,8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 3: 663-666 (1993)
Article DOI: 10.1016/S0960-894X(01)81249-X
BindingDB Entry DOI: 10.7270/Q2X92BS9 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018514

(CHEMBL3290663)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O3/c12-4-2-14-11(13)7-8(4)16(3-15-7)5-1-6(17)10(19)9(5)18/h2-3,5-6,9-10,17-19H,1H2,(H2,13,14)/t5-,6+,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018529
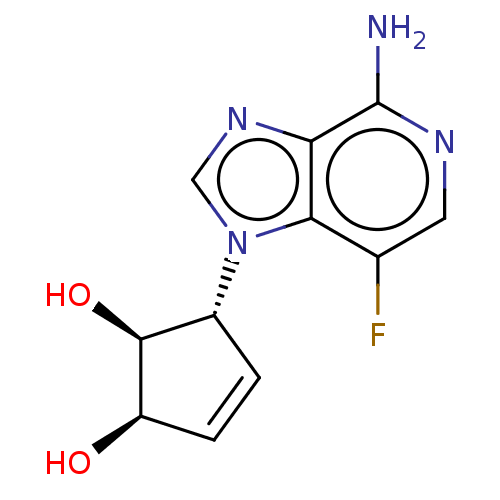
(CHEMBL3290665)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |r,c:14| Show InChI InChI=1S/C11H11FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h1-4,6-7,10,17-18H,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50316222
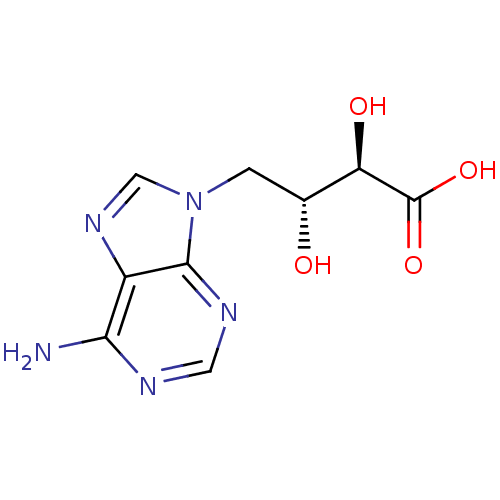
(CHEMBL1095280 | D-ERITADENINE | eritadenine)Show SMILES Nc1ncnc2n(C[C@@H](O)[C@@H](O)C(O)=O)cnc12 |r| Show InChI InChI=1S/C9H11N5O4/c10-7-5-8(12-2-11-7)14(3-13-5)1-4(15)6(16)9(17)18/h2-4,6,15-16H,1H2,(H,17,18)(H2,10,11,12)/t4-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston College
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase in intact rat hepatocytes |
Bioorg Med Chem 17: 6707-14 (2009)
Article DOI: 10.1016/j.bmc.2009.07.061
BindingDB Entry DOI: 10.7270/Q2JD4X0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50281612
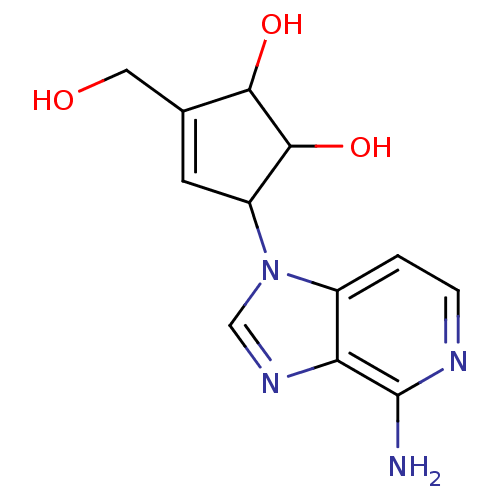
(5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-3-hydroxyme...)Show InChI InChI=1S/C12H14N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-3,5,8,10-11,17-19H,4H2,(H2,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 3: 663-666 (1993)
Article DOI: 10.1016/S0960-894X(01)81249-X
BindingDB Entry DOI: 10.7270/Q2X92BS9 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018509
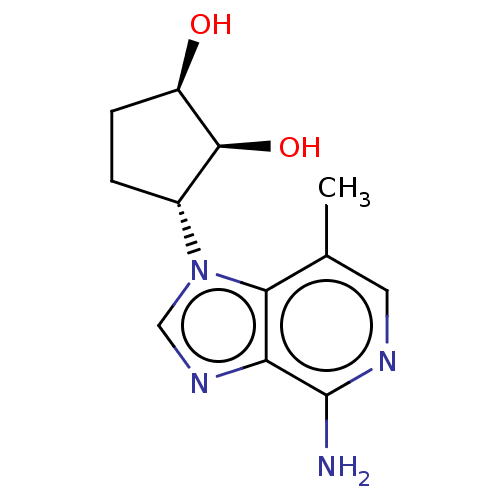
(CHEMBL3290659)Show SMILES Cc1cnc(N)c2ncn([C@@H]3CC[C@@H](O)[C@H]3O)c12 |r| Show InChI InChI=1S/C12H16N4O2/c1-6-4-14-12(13)9-10(6)16(5-15-9)7-2-3-8(17)11(7)18/h4-5,7-8,11,17-18H,2-3H2,1H3,(H2,13,14)/t7-,8-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018507

(CHEMBL3290657)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13FN4O2/c12-5-3-14-11(13)8-9(5)16(4-15-8)6-1-2-7(17)10(6)18/h3-4,6-7,10,17-18H,1-2H2,(H2,13,14)/t6-,7-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50029130

(Adenosine dialdehyde | CHEMBL165876)Show InChI InChI=1S/C10H11N5O4/c11-9-8-10(13-4-12-9)15(5-14-8)7(3-18)19-6(1-16)2-17/h1,3-7,17H,2H2,(H2,11,12,13)/t6-,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-homocysteine (AdoHcy)hydrolase from rabbit erythrocytes |
J Med Chem 31: 1798-804 (1988)
BindingDB Entry DOI: 10.7270/Q2VX0H4P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018512
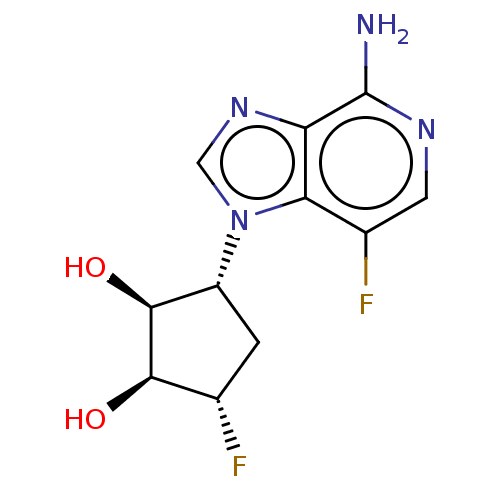
(CHEMBL3290662)Show SMILES Nc1ncc(F)c2n(cnc12)[C@@H]1C[C@H](F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12F2N4O2/c12-4-1-6(10(19)9(4)18)17-3-16-7-8(17)5(13)2-15-11(7)14/h2-4,6,9-10,18-19H,1H2,(H2,14,15)/t4-,6+,9+,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050874
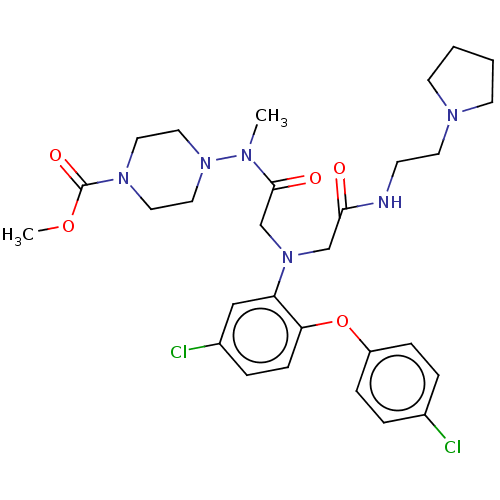
(CHEMBL3322550)Show SMILES COC(=O)N1CCN(CC1)N(C)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H38Cl2N6O5/c1-33(37-17-15-35(16-18-37)29(40)41-2)28(39)21-36(20-27(38)32-11-14-34-12-3-4-13-34)25-19-23(31)7-10-26(25)42-24-8-5-22(30)6-9-24/h5-10,19H,3-4,11-18,20-21H2,1-2H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050874

(CHEMBL3322550)Show SMILES COC(=O)N1CCN(CC1)N(C)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H38Cl2N6O5/c1-33(37-17-15-35(16-18-37)29(40)41-2)28(39)21-36(20-27(38)32-11-14-34-12-3-4-13-34)25-19-23(31)7-10-26(25)42-24-8-5-22(30)6-9-24/h5-10,19H,3-4,11-18,20-21H2,1-2H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050875
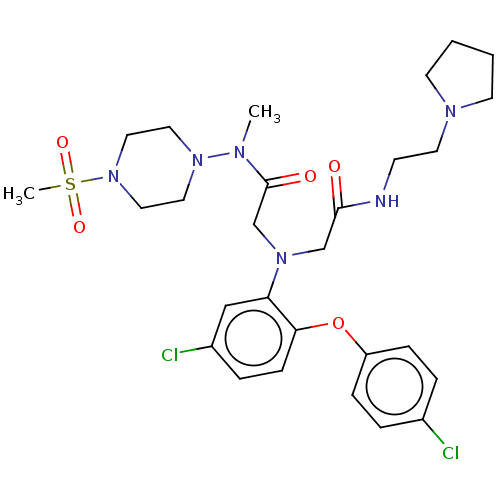
(CHEMBL3322549)Show SMILES CN(N1CCN(CC1)S(C)(=O)=O)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H38Cl2N6O5S/c1-32(35-15-17-36(18-16-35)42(2,39)40)28(38)21-34(20-27(37)31-11-14-33-12-3-4-13-33)25-19-23(30)7-10-26(25)41-24-8-5-22(29)6-9-24/h5-10,19H,3-4,11-18,20-21H2,1-2H3,(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50105907
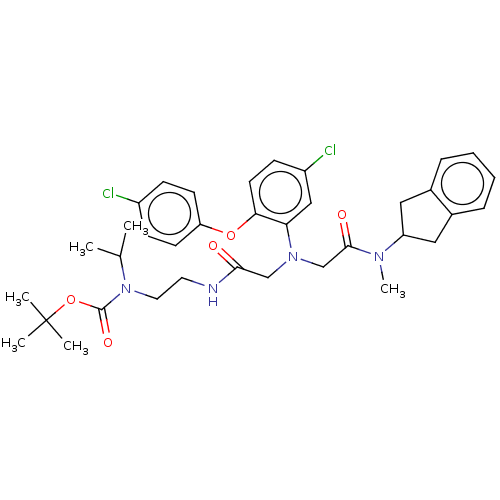
(CHEMBL3597818)Show SMILES CC(C)N(CCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1)C(=O)OC(C)(C)C Show InChI InChI=1S/C36H44Cl2N4O5/c1-24(2)42(35(45)47-36(3,4)5)18-17-39-33(43)22-41(23-34(44)40(6)29-19-25-9-7-8-10-26(25)20-29)31-21-28(38)13-16-32(31)46-30-14-11-27(37)12-15-30/h7-16,21,24,29H,17-20,22-23H2,1-6H3,(H,39,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050868
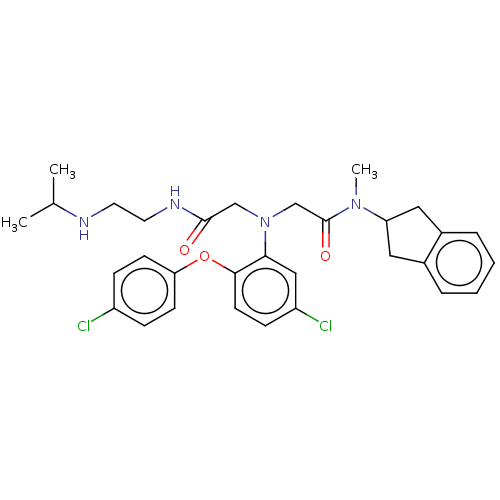
(CHEMBL3322556)Show SMILES CC(C)NCCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-21(2)34-14-15-35-30(38)19-37(20-31(39)36(3)26-16-22-6-4-5-7-23(22)17-26)28-18-25(33)10-13-29(28)40-27-11-8-24(32)9-12-27/h4-13,18,21,26,34H,14-17,19-20H2,1-3H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050875

(CHEMBL3322549)Show SMILES CN(N1CCN(CC1)S(C)(=O)=O)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H38Cl2N6O5S/c1-32(35-15-17-36(18-16-35)42(2,39)40)28(38)21-34(20-27(37)31-11-14-33-12-3-4-13-33)25-19-23(30)7-10-26(25)41-24-8-5-22(29)6-9-24/h5-10,19H,3-4,11-18,20-21H2,1-2H3,(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Mus musculus) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050884
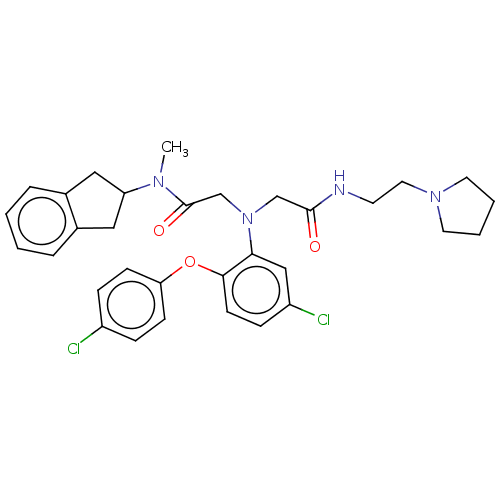
(CHEMBL3322540)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C32H36Cl2N4O3/c1-36(27-18-23-6-2-3-7-24(23)19-27)32(40)22-38(21-31(39)35-14-17-37-15-4-5-16-37)29-20-26(34)10-13-30(29)41-28-11-8-25(33)9-12-28/h2-3,6-13,20,27H,4-5,14-19,21-22H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050884

(CHEMBL3322540)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C32H36Cl2N4O3/c1-36(27-18-23-6-2-3-7-24(23)19-27)32(40)22-38(21-31(39)35-14-17-37-15-4-5-16-37)29-20-26(34)10-13-30(29)41-28-11-8-25(33)9-12-28/h2-3,6-13,20,27H,4-5,14-19,21-22H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006218

((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...)Show InChI InChI=1S/C10H13N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h3-6,8,16-17H,1-2H2,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 mins |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50105908
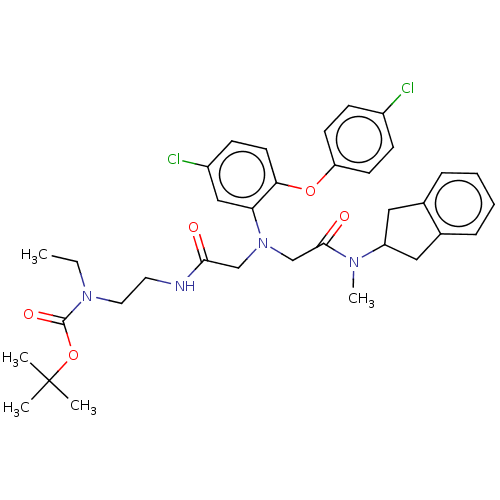
(CHEMBL3597817)Show SMILES CCN(CCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1)C(=O)OC(C)(C)C Show InChI InChI=1S/C35H42Cl2N4O5/c1-6-40(34(44)46-35(2,3)4)18-17-38-32(42)22-41(23-33(43)39(5)28-19-24-9-7-8-10-25(24)20-28)30-21-27(37)13-16-31(30)45-29-14-11-26(36)12-15-29/h7-16,21,28H,6,17-20,22-23H2,1-5H3,(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050869
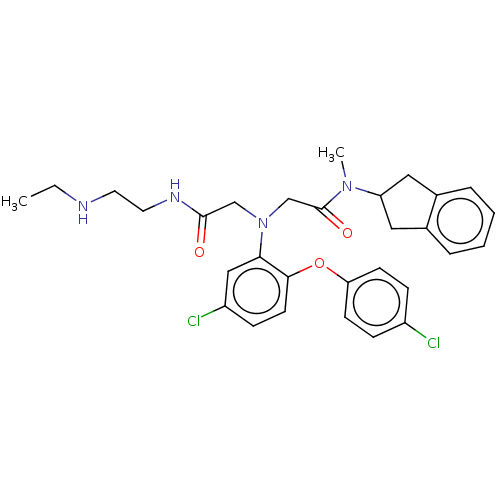
(CHEMBL3322555)Show SMILES CCNCCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H34Cl2N4O3/c1-3-33-14-15-34-29(37)19-36(20-30(38)35(2)25-16-21-6-4-5-7-22(21)17-25)27-18-24(32)10-13-28(27)39-26-11-8-23(31)9-12-26/h4-13,18,25,33H,3,14-17,19-20H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050870

(CHEMBL3322554)Show SMILES CNCCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C29H32Cl2N4O3/c1-32-13-14-33-28(36)18-35(19-29(37)34(2)24-15-20-5-3-4-6-21(20)16-24)26-17-23(31)9-12-27(26)38-25-10-7-22(30)8-11-25/h3-12,17,24,32H,13-16,18-19H2,1-2H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50105899
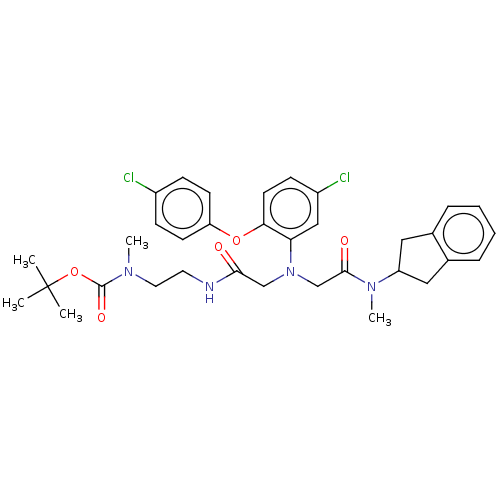
(CHEMBL3597816)Show SMILES CN(CCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1)C(=O)OC(C)(C)C Show InChI InChI=1S/C34H40Cl2N4O5/c1-34(2,3)45-33(43)38(4)17-16-37-31(41)21-40(22-32(42)39(5)27-18-23-8-6-7-9-24(23)19-27)29-20-26(36)12-15-30(29)44-28-13-10-25(35)11-14-28/h6-15,20,27H,16-19,21-22H2,1-5H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50019042
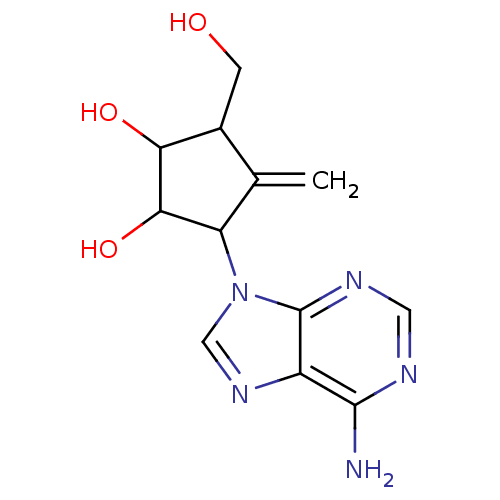
(3-(6-Amino-purin-9-yl)-5-hydroxymethyl-4-methylene...)Show InChI InChI=1S/C12H15N5O3/c1-5-6(2-18)9(19)10(20)8(5)17-4-16-7-11(13)14-3-15-12(7)17/h3-4,6,8-10,18-20H,1-2H2,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-homocysteine (AdoHcy)hydrolase from rabbit erythrocytes |
J Med Chem 31: 1798-804 (1988)
BindingDB Entry DOI: 10.7270/Q2VX0H4P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050883
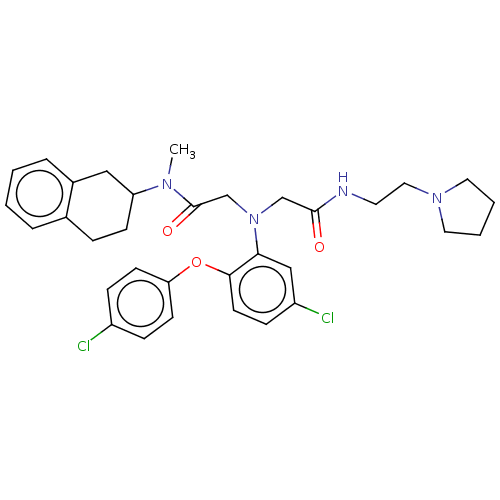
(CHEMBL3322541)Show SMILES CN(C1CCc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C33H38Cl2N4O3/c1-37(28-12-8-24-6-2-3-7-25(24)20-28)33(41)23-39(22-32(40)36-16-19-38-17-4-5-18-38)30-21-27(35)11-15-31(30)42-29-13-9-26(34)10-14-29/h2-3,6-7,9-11,13-15,21,28H,4-5,8,12,16-20,22-23H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050883

(CHEMBL3322541)Show SMILES CN(C1CCc2ccccc2C1)C(=O)CN(CC(=O)NCCN1CCCC1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C33H38Cl2N4O3/c1-37(28-12-8-24-6-2-3-7-25(24)20-28)33(41)23-39(22-32(40)36-16-19-38-17-4-5-18-38)30-21-27(35)11-15-31(30)42-29-13-9-26(34)10-14-29/h2-3,6-7,9-11,13-15,21,28H,4-5,8,12,16-20,22-23H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50018505
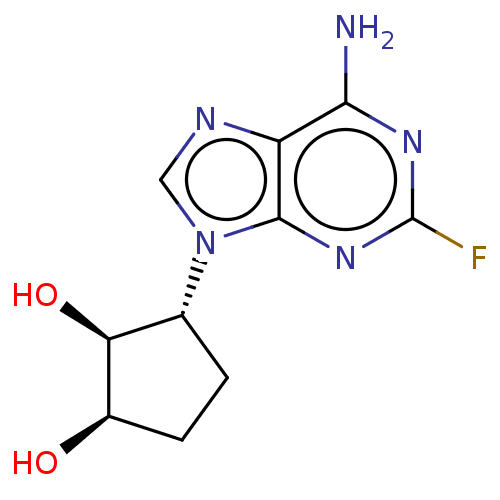
(CHEMBL3290655)Show SMILES Nc1nc(F)nc2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12FN5O2/c11-10-14-8(12)6-9(15-10)16(3-13-6)4-1-2-5(17)7(4)18/h3-5,7,17-18H,1-2H2,(H2,12,14,15)/t4-,5-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysis |
Bioorg Med Chem Lett 24: 2737-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.034
BindingDB Entry DOI: 10.7270/Q2VM4DTT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50105904
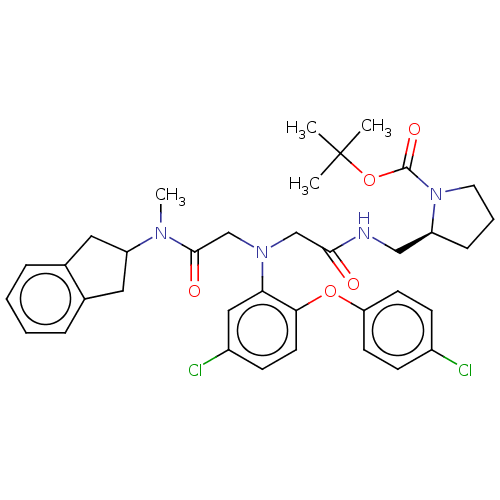
(CHEMBL3597821)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NC[C@@H]1CCCN1C(=O)OC(C)(C)C)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C36H42Cl2N4O5/c1-36(2,3)47-35(45)42-17-7-10-28(42)21-39-33(43)22-41(23-34(44)40(4)29-18-24-8-5-6-9-25(24)19-29)31-20-27(38)13-16-32(31)46-30-14-11-26(37)12-15-30/h5-6,8-9,11-16,20,28-29H,7,10,17-19,21-23H2,1-4H3,(H,39,43)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050865
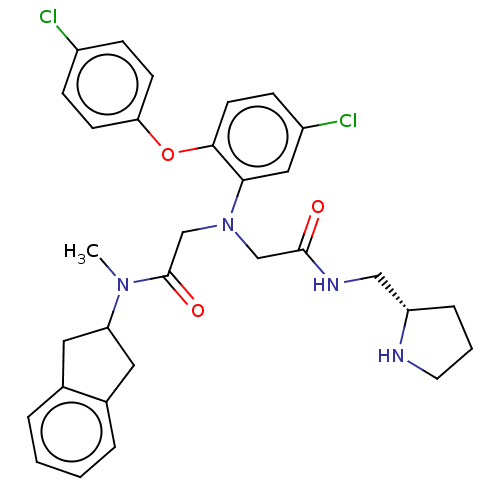
(CHEMBL3322559)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NC[C@@H]1CCCN1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C31H34Cl2N4O3/c1-36(26-15-21-5-2-3-6-22(21)16-26)31(39)20-37(19-30(38)35-18-25-7-4-14-34-25)28-17-24(33)10-13-29(28)40-27-11-8-23(32)9-12-27/h2-3,5-6,8-13,17,25-26,34H,4,7,14-16,18-20H2,1H3,(H,35,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050871
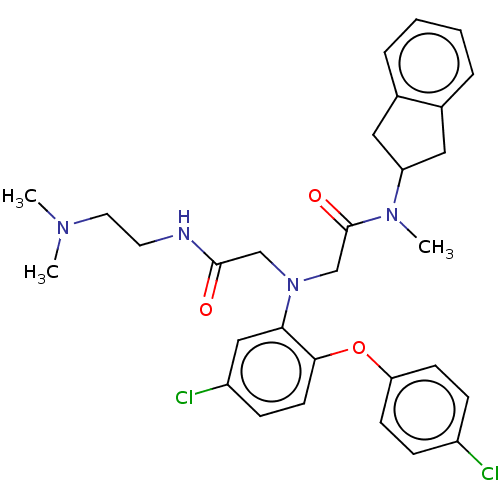
(CHEMBL3322553)Show SMILES CN(C)CCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H34Cl2N4O3/c1-34(2)15-14-33-29(37)19-36(20-30(38)35(3)25-16-21-6-4-5-7-22(21)17-25)27-18-24(32)10-13-28(27)39-26-11-8-23(31)9-12-26/h4-13,18,25H,14-17,19-20H2,1-3H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050871

(CHEMBL3322553)Show SMILES CN(C)CCNC(=O)CN(CC(=O)N(C)C1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C30H34Cl2N4O3/c1-34(2)15-14-33-29(37)19-36(20-30(38)35(3)25-16-21-6-4-5-7-22(21)17-25)27-18-24(32)10-13-28(27)39-26-11-8-23(31)9-12-26/h4-13,18,25H,14-17,19-20H2,1-3H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by HPLC analysis |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50105902
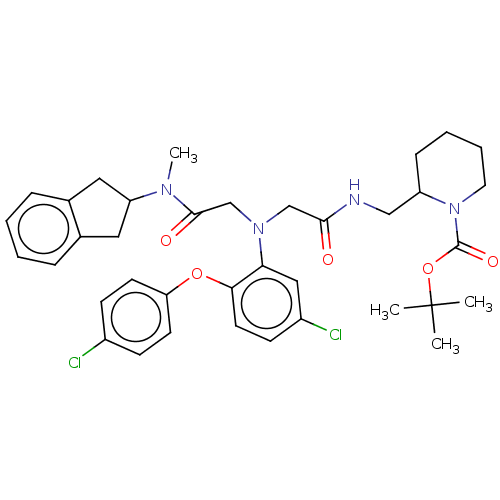
(CHEMBL3597823)Show SMILES CN(C1Cc2ccccc2C1)C(=O)CN(CC(=O)NCC1CCCCN1C(=O)OC(C)(C)C)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C37H44Cl2N4O5/c1-37(2,3)48-36(46)43-18-8-7-11-29(43)22-40-34(44)23-42(24-35(45)41(4)30-19-25-9-5-6-10-26(25)20-30)32-21-28(39)14-17-33(32)47-31-15-12-27(38)13-16-31/h5-6,9-10,12-17,21,29-30H,7-8,11,18-20,22-24H2,1-4H3,(H,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human S-adenosyl-L-homocysteine hydrolase assessed as hydrolysis of AdoHcy after 8 mins by HPLC analysis |
Bioorg Med Chem 23: 4952-69 (2015)
Article DOI: 10.1016/j.bmc.2015.05.018
BindingDB Entry DOI: 10.7270/Q29888ST |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data