 Found 137 hits of ki data for polymerid = 50000561,50002182,50002390,50005075
Found 137 hits of ki data for polymerid = 50000561,50002182,50002390,50005075 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50096906
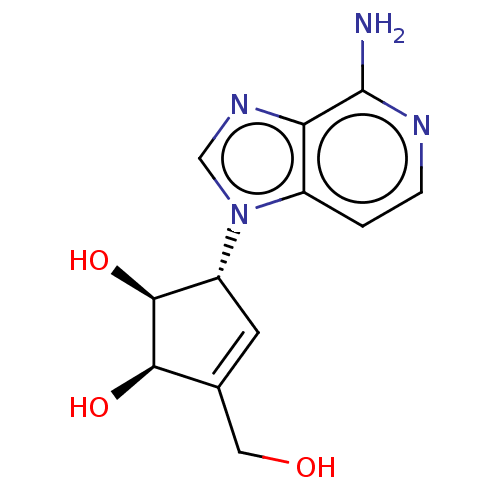
(CHEMBL154745 | US10227373, Compound D-3-Deazaisone...)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C12H14N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-3,5,8,10-11,17-19H,4H2,(H2,13,14)/t8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50240434
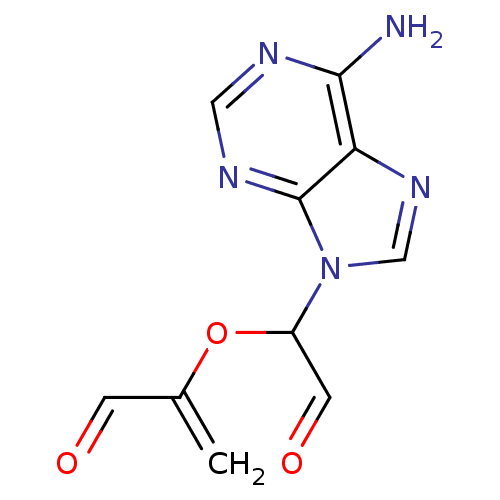
(2-[1-(6-Amino-purin-9-yl)-2-oxo-ethoxy]-propenal |...)Show InChI InChI=1S/C10H9N5O3/c1-6(2-16)18-7(3-17)15-5-14-8-9(11)12-4-13-10(8)15/h2-5,7H,1H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of S-adenosyl homocysteine (SAH) hydrolase . |
Bioorg Med Chem Lett 6: 1381-1386 (1996)
Article DOI: 10.1016/0960-894X(96)00234-X
BindingDB Entry DOI: 10.7270/Q29P31NC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50050862
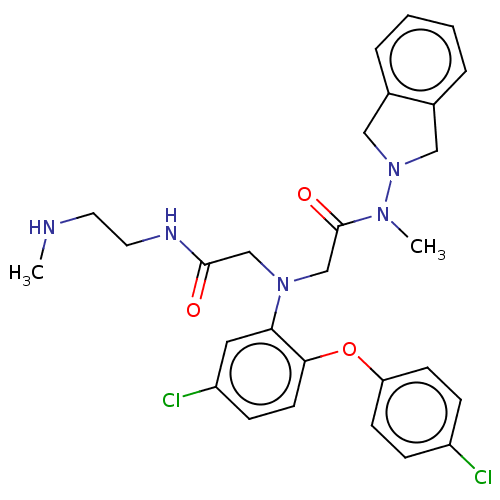
(CHEMBL3322562)Show SMILES Cl.CNCCNC(=O)CN(CC(=O)N(C)N1Cc2ccccc2C1)c1cc(Cl)ccc1Oc1ccc(Cl)cc1 Show InChI InChI=1S/C28H31Cl2N5O3.ClH/c1-31-13-14-32-27(36)18-34(19-28(37)33(2)35-16-20-5-3-4-6-21(20)17-35)25-15-23(30)9-12-26(25)38-24-10-7-22(29)8-11-24;/h3-12,15,31H,13-14,16-19H2,1-2H3,(H,32,36);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant S-adenosyl-L-homocysteine hydrolase assessed as AdoHcy hydrolysis activity by Lineweaver-Burk plot |
Bioorg Med Chem Lett 24: 4336-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.008
BindingDB Entry DOI: 10.7270/Q2377BCP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Mus musculus) | BDBM50006222
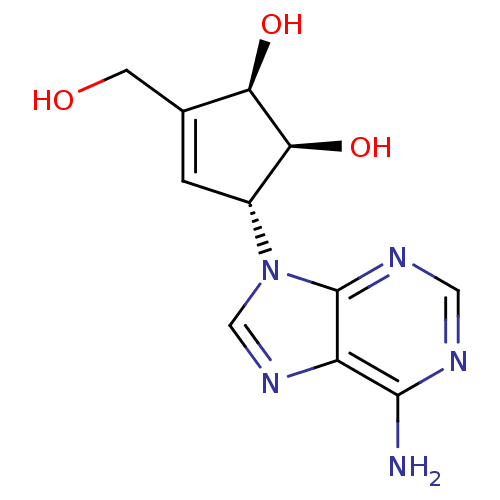
((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was tested against L929 cells AdoHcy hydrolase activity |
J Med Chem 37: 551-4 (1994)
BindingDB Entry DOI: 10.7270/Q2DJ5DQ4 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50029130

(Adenosine dialdehyde | CHEMBL165876)Show InChI InChI=1S/C10H11N5O4/c11-9-8-10(13-4-12-9)15(5-14-8)7(3-18)19-6(1-16)2-17/h1,3-7,17H,2H2,(H2,11,12,13)/t6-,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of S-adenosyl homocysteine (SAH) hydrolase . |
Bioorg Med Chem Lett 6: 1381-1386 (1996)
Article DOI: 10.1016/0960-894X(96)00234-X
BindingDB Entry DOI: 10.7270/Q29P31NC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase |
J Med Chem 31: 500-3 (1988)
BindingDB Entry DOI: 10.7270/Q28P61Q3 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy) rate of inactivation by NpcA |
J Med Chem 35: 1782-91 (1992)
BindingDB Entry DOI: 10.7270/Q23J3BW0 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50367250

(3-DEAZAARISTEROMYCIN A | CHEMBL268272)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C12H16N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-2,5-6,8,10-11,17-19H,3-4H2,(H2,13,14)/t6-,8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Reversible inhibition of rat liver S- adenosyl-L-homocysteine hydrolase using SAH as substrate by spectrophotometric method |
J Med Chem 63: 6315-6386 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01877
BindingDB Entry DOI: 10.7270/Q2NG4V6W |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Mus musculus) | BDBM50034176
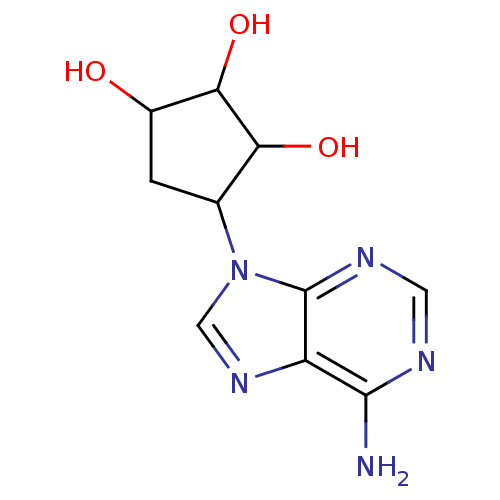
(4-(6-Amino-purin-9-yl)-cyclopentane-1,2,3-triol | ...)Show InChI InChI=1S/C10H13N5O3/c11-9-6-10(13-2-12-9)15(3-14-6)4-1-5(16)8(18)7(4)17/h2-5,7-8,16-18H,1H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was tested against L929 cells AdoHcy hydrolase activity |
J Med Chem 37: 551-4 (1994)
BindingDB Entry DOI: 10.7270/Q2DJ5DQ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006218
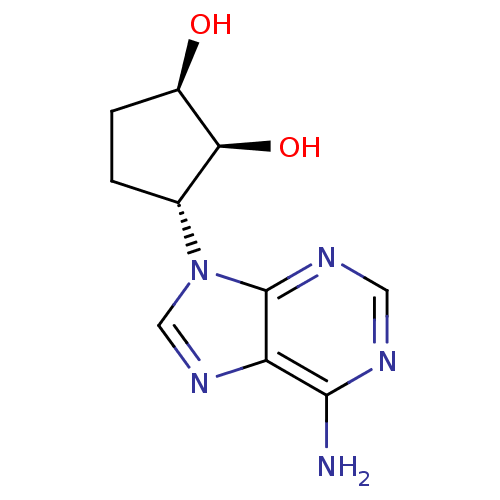
((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...)Show InChI InChI=1S/C10H13N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h3-6,8,16-17H,1-2H2,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). |
J Med Chem 35: 1782-91 (1992)
BindingDB Entry DOI: 10.7270/Q23J3BW0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006220
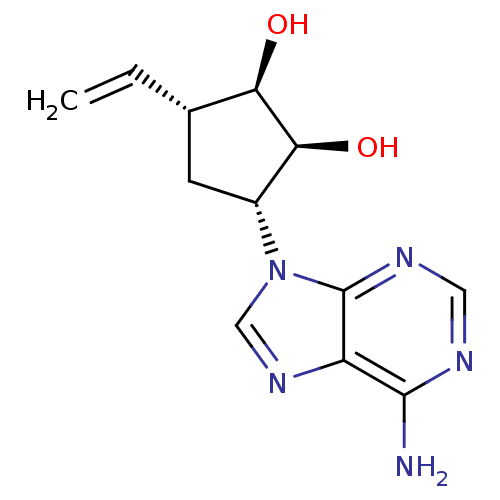
(3-(6-Amino-purin-9-yl)-5-vinyl-cyclopentane-1,2-di...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](C=C)[C@@H](O)[C@H]1O Show InChI InChI=1S/C12H15N5O2/c1-2-6-3-7(10(19)9(6)18)17-5-16-8-11(13)14-4-15-12(8)17/h2,4-7,9-10,18-19H,1,3H2,(H2,13,14,15)/t6-,7+,9+,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). |
J Med Chem 35: 1782-91 (1992)
BindingDB Entry DOI: 10.7270/Q23J3BW0 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006221
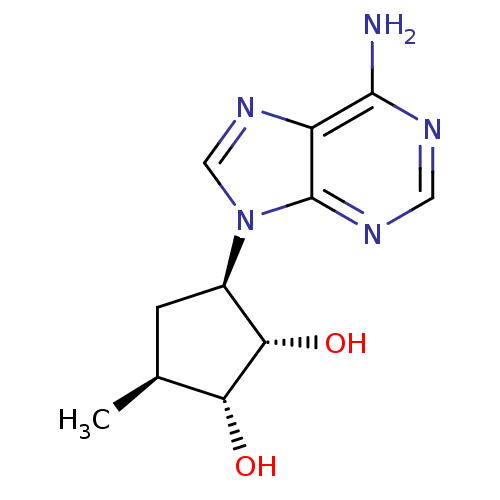
(3-(6-Amino-purin-9-yl)-5-methyl-cyclopentane-1,2-d...)Show SMILES C[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C11H15N5O2/c1-5-2-6(9(18)8(5)17)16-4-15-7-10(12)13-3-14-11(7)16/h3-6,8-9,17-18H,2H2,1H3,(H2,12,13,14)/t5-,6+,8+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). |
J Med Chem 35: 1782-91 (1992)
BindingDB Entry DOI: 10.7270/Q23J3BW0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50405655

(CHEMBL147260)Show SMILES Nc1nccc2n(cnc12)C1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C11H12N4O2/c12-11-9-6(3-4-13-11)15(5-14-9)7-1-2-8(16)10(7)17/h1-5,7-8,10,16-17H,(H2,12,13)/t7?,8-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase |
J Med Chem 31: 500-3 (1988)
BindingDB Entry DOI: 10.7270/Q28P61Q3 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50051436
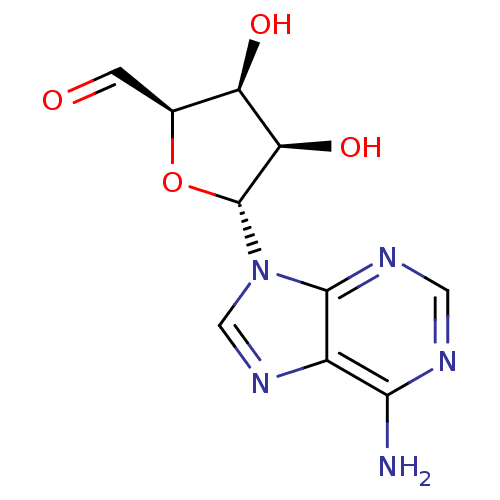
((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051436

((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase |
J Med Chem 39: 2347-53 (1996)
Article DOI: 10.1021/jm950916u
BindingDB Entry DOI: 10.7270/Q20Z72CP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50280299
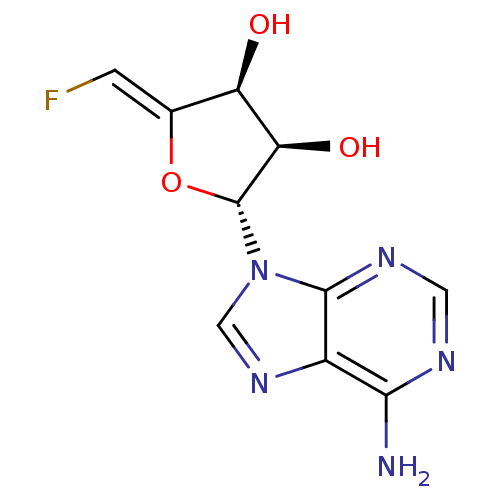
((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O\C(=C/F)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H10FN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h1-3,6-7,10,17-18H,(H2,12,13,14)/b4-1-/t6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase |
Bioorg Med Chem Lett 2: 1741-1744 (1992)
Article DOI: 10.1016/S0960-894X(00)80467-9
BindingDB Entry DOI: 10.7270/Q21Z44B7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50046747
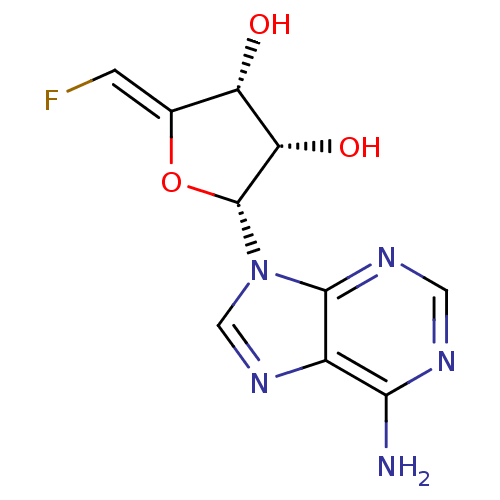
(2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O\C(=C/F)[C@H](O)[C@@H]1O Show InChI InChI=1S/C10H10FN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h1-3,6-7,10,17-18H,(H2,12,13,14)/b4-1-/t6-,7-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435
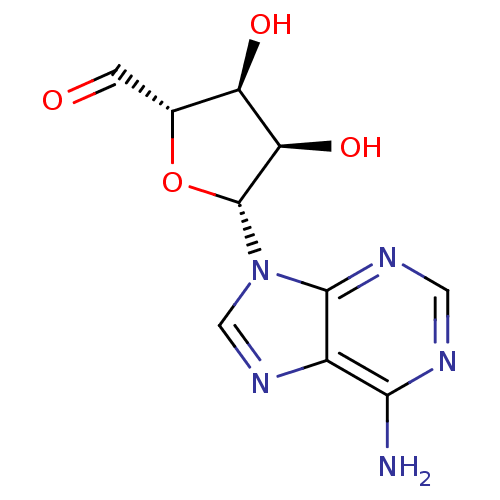
(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50280301

((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O\C(=C\F)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H10FN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h1-3,6-7,10,17-18H,(H2,12,13,14)/b4-1+/t6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase |
Bioorg Med Chem Lett 2: 1741-1744 (1992)
Article DOI: 10.1016/S0960-894X(00)80467-9
BindingDB Entry DOI: 10.7270/Q21Z44B7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50046748
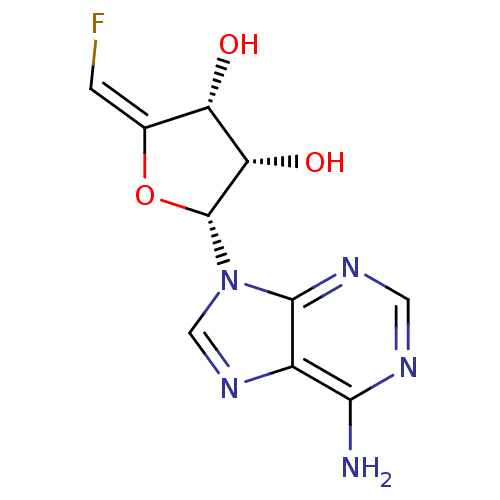
(2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O\C(=C\F)[C@H](O)[C@@H]1O Show InChI InChI=1S/C10H10FN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h1-3,6-7,10,17-18H,(H2,12,13,14)/b4-1+/t6-,7-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006215
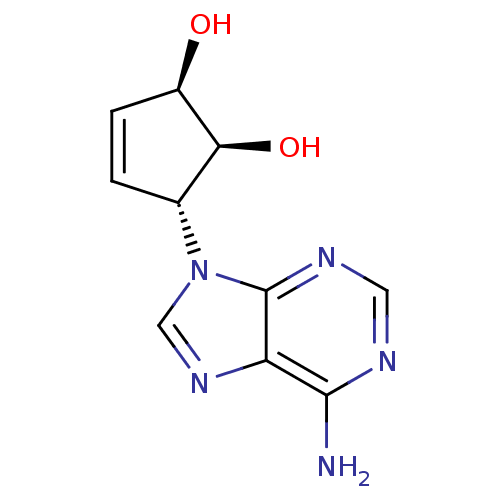
((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C10H11N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h1-6,8,16-17H,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase |
J Med Chem 31: 500-3 (1988)
BindingDB Entry DOI: 10.7270/Q28P61Q3 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase |
J Med Chem 39: 2347-53 (1996)
Article DOI: 10.1021/jm950916u
BindingDB Entry DOI: 10.7270/Q20Z72CP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051436

((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006215

((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C10H11N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h1-6,8,16-17H,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). |
J Med Chem 35: 1782-91 (1992)
BindingDB Entry DOI: 10.7270/Q23J3BW0 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Mus musculus) | BDBM50008288
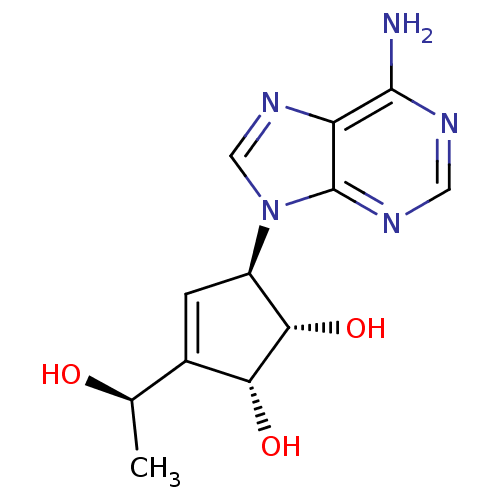
((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((R)-1-hydroxy...)Show SMILES C[C@@H](O)C1=C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |t:3| Show InChI InChI=1S/C12H15N5O3/c1-5(18)6-2-7(10(20)9(6)19)17-4-16-8-11(13)14-3-15-12(8)17/h2-5,7,9-10,18-20H,1H3,(H2,13,14,15)/t5-,7-,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase |
J Med Chem 35: 324-31 (1992)
BindingDB Entry DOI: 10.7270/Q2RX9B1R |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50280300
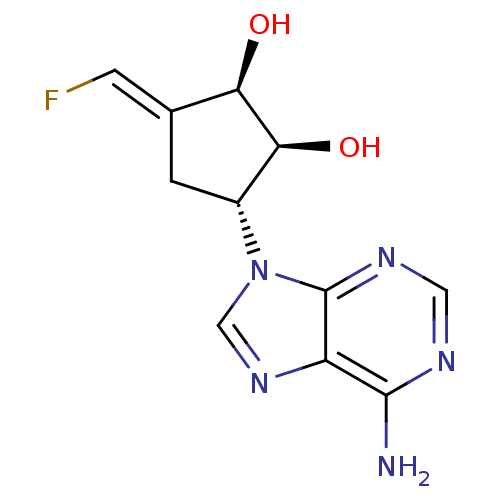
((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C\C(=C/F)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H12FN5O2/c12-2-5-1-6(9(19)8(5)18)17-4-16-7-10(13)14-3-15-11(7)17/h2-4,6,8-9,18-19H,1H2,(H2,13,14,15)/b5-2+/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase |
Bioorg Med Chem Lett 2: 1741-1744 (1992)
Article DOI: 10.1016/S0960-894X(00)80467-9
BindingDB Entry DOI: 10.7270/Q21Z44B7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023889
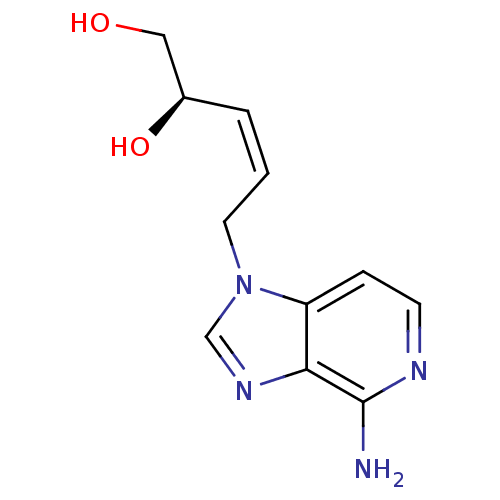
(5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-pent-3-ene-...)Show InChI InChI=1S/C11H14N4O2/c12-11-10-9(3-4-13-11)15(7-14-10)5-1-2-8(17)6-16/h1-4,7-8,16-17H,5-6H2,(H2,12,13)/b2-1-/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369258
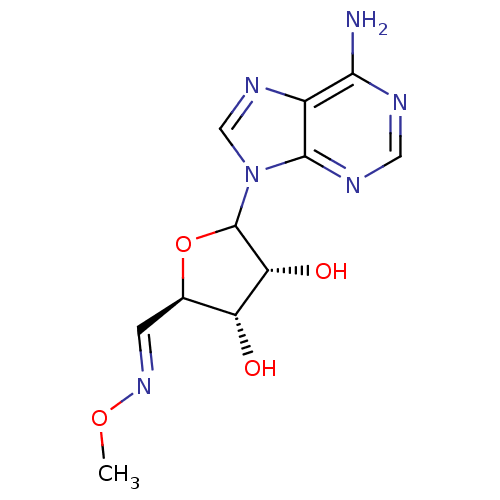
(CHEMBL606276)Show SMILES CO\N=C\[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14N6O4/c1-20-16-2-5-7(18)8(19)11(21-5)17-4-15-6-9(12)13-3-14-10(6)17/h2-5,7-8,11,18-19H,1H3,(H2,12,13,14)/b16-2+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
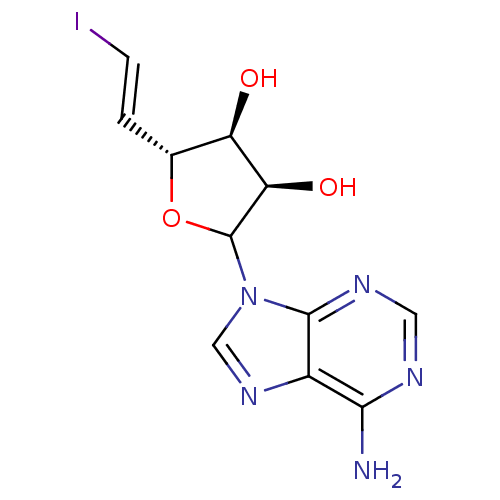
(Homo sapiens (Human)) | BDBM50368896
(CHEMBL608056)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C\I)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12IN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
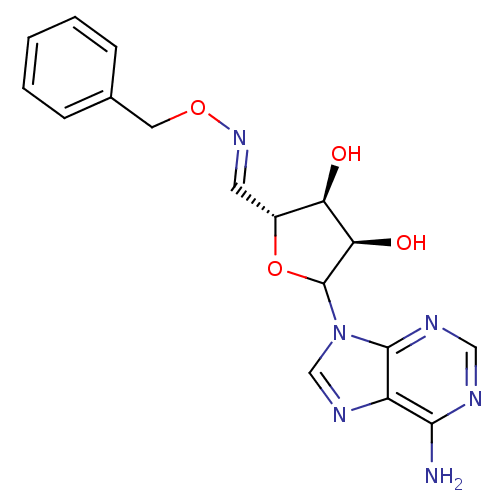
(Homo sapiens (Human)) | BDBM50369257
(CHEMBL605902)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=N\OCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H18N6O4/c18-15-12-16(20-8-19-15)23(9-21-12)17-14(25)13(24)11(27-17)6-22-26-7-10-4-2-1-3-5-10/h1-6,8-9,11,13-14,17,24-25H,7H2,(H2,18,19,20)/b22-6+/t11-,13-,14-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50088426

((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H15N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h3-6,8-9,17-19H,1-2H2,(H2,12,13,14)/t5-,6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407233
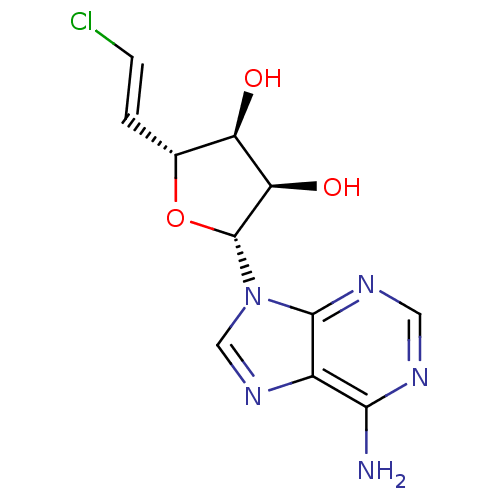
(CHEMBL2092790)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C\Cl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12ClN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023879

(2-[2-(6-Amino-purin-9-yl)-ethylidene]-propane-1,3-...)Show SMILES [#7]-c1ncnc2n(-[#6]\[#6]=[#6](/[#6]-[#8])-[#6]-[#8])cnc12 Show InChI InChI=1S/C10H13N5O2/c11-9-8-10(13-5-12-9)15(6-14-8)2-1-7(3-16)4-17/h1,5-6,16-17H,2-4H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369255

(CHEMBL605900)Show SMILES CCO\N=C\[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H16N6O4/c1-2-21-17-3-6-8(19)9(20)12(22-6)18-5-16-7-10(13)14-4-15-11(7)18/h3-6,8-9,12,19-20H,2H2,1H3,(H2,13,14,15)/b17-3+/t6-,8-,9-,12?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50280298

((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C\C(=C\F)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H12FN5O2/c12-2-5-1-6(9(19)8(5)18)17-4-16-7-10(13)14-3-15-11(7)17/h2-4,6,8-9,18-19H,1H2,(H2,13,14,15)/b5-2-/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase |
Bioorg Med Chem Lett 2: 1741-1744 (1992)
Article DOI: 10.1016/S0960-894X(00)80467-9
BindingDB Entry DOI: 10.7270/Q21Z44B7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50011148
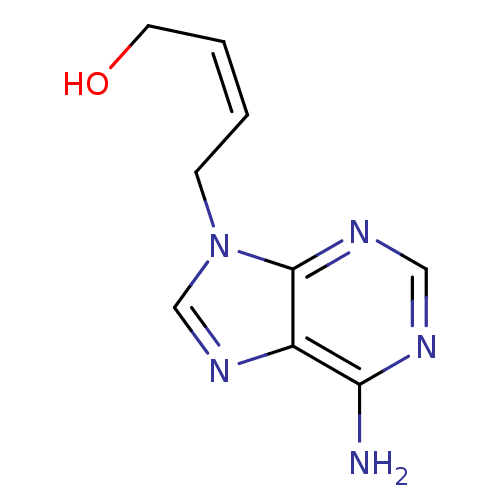
((Z)-4-(6-Amino-purin-9-yl)-but-2-en-1-ol | 4-(6-Am...)Show InChI InChI=1S/C9H11N5O/c10-8-7-9(12-5-11-8)14(6-13-7)3-1-2-4-15/h1-2,5-6,15H,3-4H2,(H2,10,11,12)/b2-1- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407232

(CHEMBL2092789)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C\Br)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12BrN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Mus musculus) | BDBM50006223
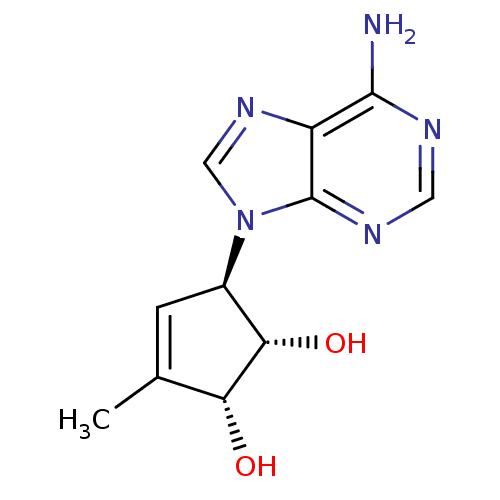
(5-(6-Amino-purin-9-yl)-3-methyl-cyclopent-3-ene-1,...)Show SMILES CC1=C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |t:1| Show InChI InChI=1S/C11H13N5O2/c1-5-2-6(9(18)8(5)17)16-4-15-7-10(12)13-3-14-11(7)16/h2-4,6,8-9,17-18H,1H3,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase |
J Med Chem 35: 324-31 (1992)
BindingDB Entry DOI: 10.7270/Q2RX9B1R |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023885

(4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-yn-1-...)Show InChI InChI=1S/C10H10N4O/c11-10-9-8(3-4-12-10)14(7-13-9)5-1-2-6-15/h3-4,7,15H,5-6H2,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50135288

((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O3/c11-9-6-10(13-2-12-9)15(3-14-6)4-1-5(16)8(18)7(4)17/h2-5,7-8,16-18H,1H2,(H2,11,12,13)/t4-,5+,7+,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase |
Bioorg Med Chem Lett 13: 3963-5 (2003)
BindingDB Entry DOI: 10.7270/Q2125S2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023886
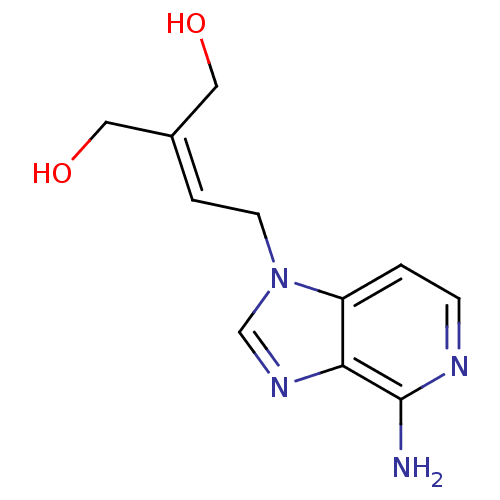
(2-[2-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-ethylide...)Show SMILES [#7]-c1nccc2n(-[#6]\[#6]=[#6](/[#6]-[#8])-[#6]-[#8])cnc12 Show InChI InChI=1S/C11H14N4O2/c12-11-10-9(1-3-13-11)15(7-14-10)4-2-8(5-16)6-17/h1-3,7,16-17H,4-6H2,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023887

(4-(6-Amino-purin-9-yl)-2-methyl-but-2-en-1-ol | CH...)Show InChI InChI=1S/C10H13N5O/c1-7(4-16)2-3-15-6-14-8-9(11)12-5-13-10(8)15/h2,5-6,16H,3-4H2,1H3,(H2,11,12,13)/b7-2- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Plasmodium falciparum 3D7) | BDBM50135288

((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O3/c11-9-6-10(13-2-12-9)15(3-14-6)4-1-5(16)8(18)7(4)17/h2-5,7-8,16-18H,1H2,(H2,11,12,13)/t4-,5+,7+,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase |
Bioorg Med Chem Lett 13: 3963-5 (2003)
BindingDB Entry DOI: 10.7270/Q2125S2X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023882

(5-(6-Amino-purin-9-yl)-pent-3-ene-1,2-diol | CHEMB...)Show InChI InChI=1S/C10H13N5O2/c11-9-8-10(13-5-12-9)15(6-14-8)3-1-2-7(17)4-16/h1-2,5-7,16-17H,3-4H2,(H2,11,12,13)/b2-1-/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023877
(4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-en-1-...)Show InChI InChI=1S/C10H12N4O/c11-10-9-8(3-4-12-10)14(7-13-9)5-1-2-6-15/h1-4,7,15H,5-6H2,(H2,11,12)/b2-1- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50408149
(CHEMBL2093112)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CN=O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C10H12N6O4/c11-8-5-9(13-2-12-8)16(3-14-5)10-7(18)6(17)4(20-10)1-15-19/h2-4,6-7,10,17-18H,1H2,(H2,11,12,13)/t4-,6-,7+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data